Jog Raj1*, Simon Jackson2, and Marko Vasiljević1
1PATENT CO DOO., Vlade Ćetkovića 1A, 24 211, Mišićevo, Serbia
2Molendotech Ltd., Brixham Laboratory, Freshwater Quarry, Brixham, Devon, UK
*Corresponding author: [email protected]
Feed ingredients and animal feed can be contaminated with various chemically and biologically derived residues, including dioxins, heavy metals, microbes, endotoxins, and mycotoxins. Among these contaminants little is understood about endotoxins. In this article the following areas on endotoxins are covered: 1. Endotoxin structure and effects on animals 2. Prevention of endotoxin absorption 3. Adsorption of endotoxins usin Minazel, Minazel Plus and MycoRaid
Endotoxin structure and effects on animals Endotoxins are lipopolysaccharides (LPS) found on the outer membrane of Gram-negative bacteria. They are structural components of the bacterial cell wall and they form part of the building blocks of the outer membrane of these bacteria.
⇰ Endotoxins are not inherently toxic per se in the same way that exotoxins. However, they induce strong inflammatory responses that can damage the host and may even be lethal. ⇰ Endotoxins are released into the environment when the bacteria multiply or when their cell membranes rupture through bacterial lysis.
LPS are chemically composed of an outer O-polysaccharide chain (O-antigen), an inner R-polysaccharide chain and lipid A (Figure 1). Figure 1. Schematic structure of the LPS from E. coli (Adapted from Abate et al., Journal of Medical Microbiology, 2017).
![]() The O-antigen varies between different bacterial strains and is the basis of the serological typing of Gram-negative bacteria such as E. coli.
The O-antigen varies between different bacterial strains and is the basis of the serological typing of Gram-negative bacteria such as E. coli.![]() Lipid A is the most bioactive part of the molecule and it is a more conserved structure. However, there may be structural differences between bacterial strains and species that alter the way host cells respond to them.
Lipid A is the most bioactive part of the molecule and it is a more conserved structure. However, there may be structural differences between bacterial strains and species that alter the way host cells respond to them. Alterations to the Lipid A may allow pathogenic bacteria to evade the host immune response.
Alterations to the Lipid A may allow pathogenic bacteria to evade the host immune response.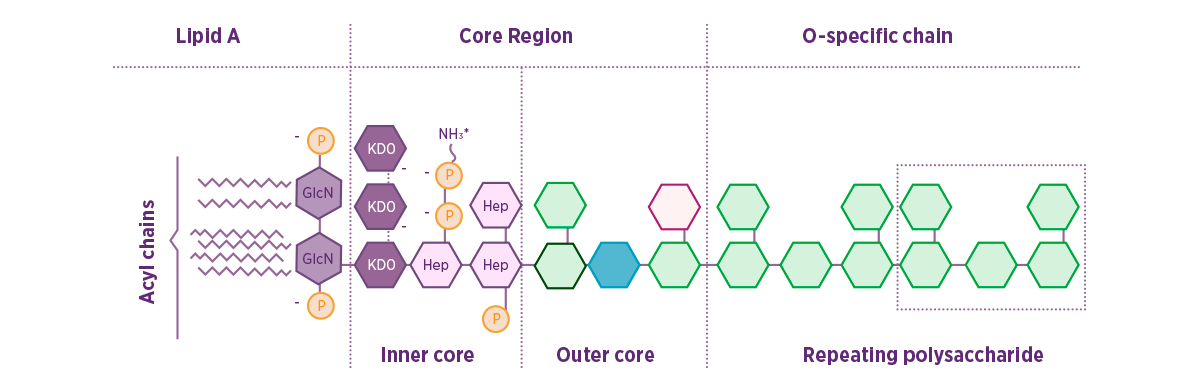
Endotoxins vs. Exotoxins Both, endotoxins and exotoxins are potentially hazardous to cell cultures, animals, and humans, though they elicit their effects through different mechanisms.







 Micotoxicosis prevention
Micotoxicosis prevention