María Rodríguez-Blanco, Sonia Marín, Vicente Sanchis, Antonio J. Ramos
Applied Mycology Unit, Department of Food Technology, ETSEA-University of Lleida, UTPV-XaRTA, Agrotecnio, Spain.
 Mycotoxins are low molecular weight secondary metabolites produced by certain genera of filamentous fungi under environmental conditions that are favourable for their synthesis (Bennett and Klich 2003).
Mycotoxins are low molecular weight secondary metabolites produced by certain genera of filamentous fungi under environmental conditions that are favourable for their synthesis (Bennett and Klich 2003).
Contamination of raw materials used for the formulation of feed with mycotoxins is a worldwide problem that causes significant economic losses.
Furthermore, the intake of contaminated feed may lead to acute or chronic intoxication in the animals and may also contribute to its consumption by humans, due to the possible transfer of these compounds to animal products such as milk, meat or eggs (Fink-Gremmels 2008a; Pinotti et al. 2016).

 The main mycotoxins that can be found contaminating feed and feed materials are aflatoxins (AFs), deoxynivalenol (DON), fumonisins (FBs), ochratoxin A (OTA), T-2 toxin and zearalenone (ZEN).
The main mycotoxins that can be found contaminating feed and feed materials are aflatoxins (AFs), deoxynivalenol (DON), fumonisins (FBs), ochratoxin A (OTA), T-2 toxin and zearalenone (ZEN).Toxic effects of mycotoxins in dairy cows
Ruminants are considered to be relatively resistant to mycotoxins, as ruminal microorganisms are able to degrade these compounds to less toxic, or even biologically inactive, compounds at normal exposure levels (Fink-Gremmels 2008b).
However, it should be noted that the rumen’s degradation capacity can become saturated or affected by changes in the diet or as a result of metabolic diseases (Fink-Gremmels 2008b). Therefore, consumption of feed contaminated with these compounds can affect the health status of dairy cows.
Some of the negative effects caused by mycotoxins in cattle are listed in Table 1.
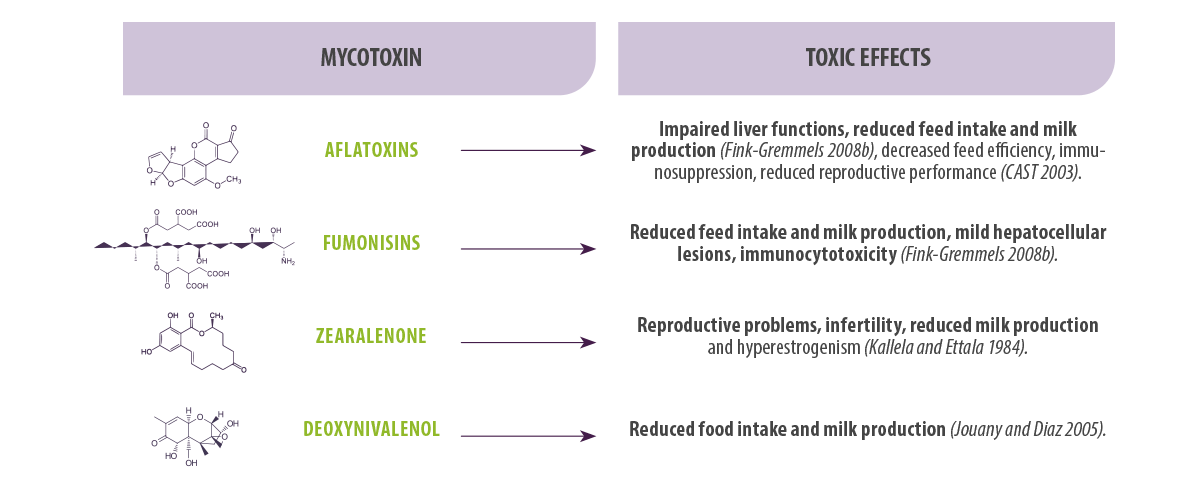
Table 1. Main toxic effects in dairy cows derived from the consumption of feed contaminated with AFs, FBs, ZEN and DON.









 Micotoxicosis prevention
Micotoxicosis prevention