BACKGROUND
BACKGROUND
Deoxynivalenol (DON), a mycotoxin produced by the fungus Fusarium graminearum, frequently contaminates crops, negatively impacting plant growth and crop yields.
![]() Its presence in food and feed poses a severe threat to human and animal health1.
Its presence in food and feed poses a severe threat to human and animal health1.
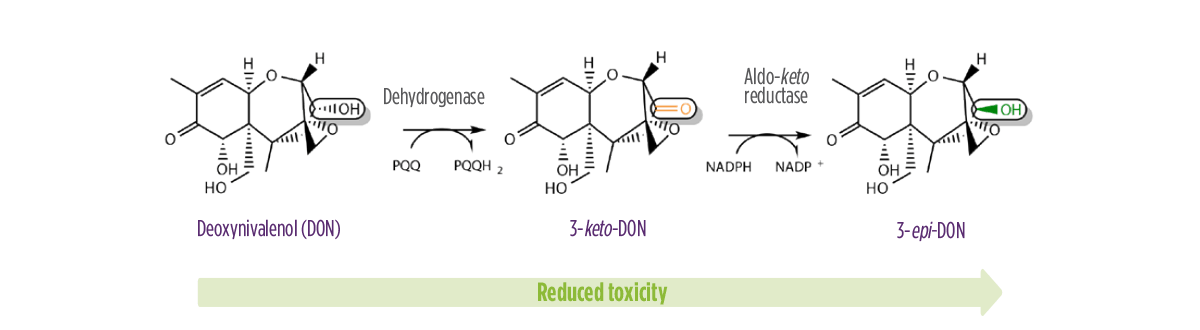
DON’s toxicity is related to the hydroxyl group at the C-3 atom, which can exist in two configurations:
- ⇰ S-configuration (DON)
- ⇰ R-configuration (3-epi-DON)
Studies have shown that 3-epi-DON is at least 50 times less toxic than DON2.
The conversion of DON into 3-epi-DON, which involves the formation of 3-keto-DON, is catalyzed by two classes of enzymes3:
- ⇰ PQQ-dependent dehydrogenases (DHs)
- ⇰ NADPH-dependent aldo-keto reductases (AKRs)
OBJECTIVE
OBJECTIVE

![]() The aim of this study was to identify and develop two robust and efficient biocatalysts with the required industrial characteristics to deactivate DON in animal feed.
The aim of this study was to identify and develop two robust and efficient biocatalysts with the required industrial characteristics to deactivate DON in animal feed.
MATERIALS & METHODS
MATERIALS & METHODS
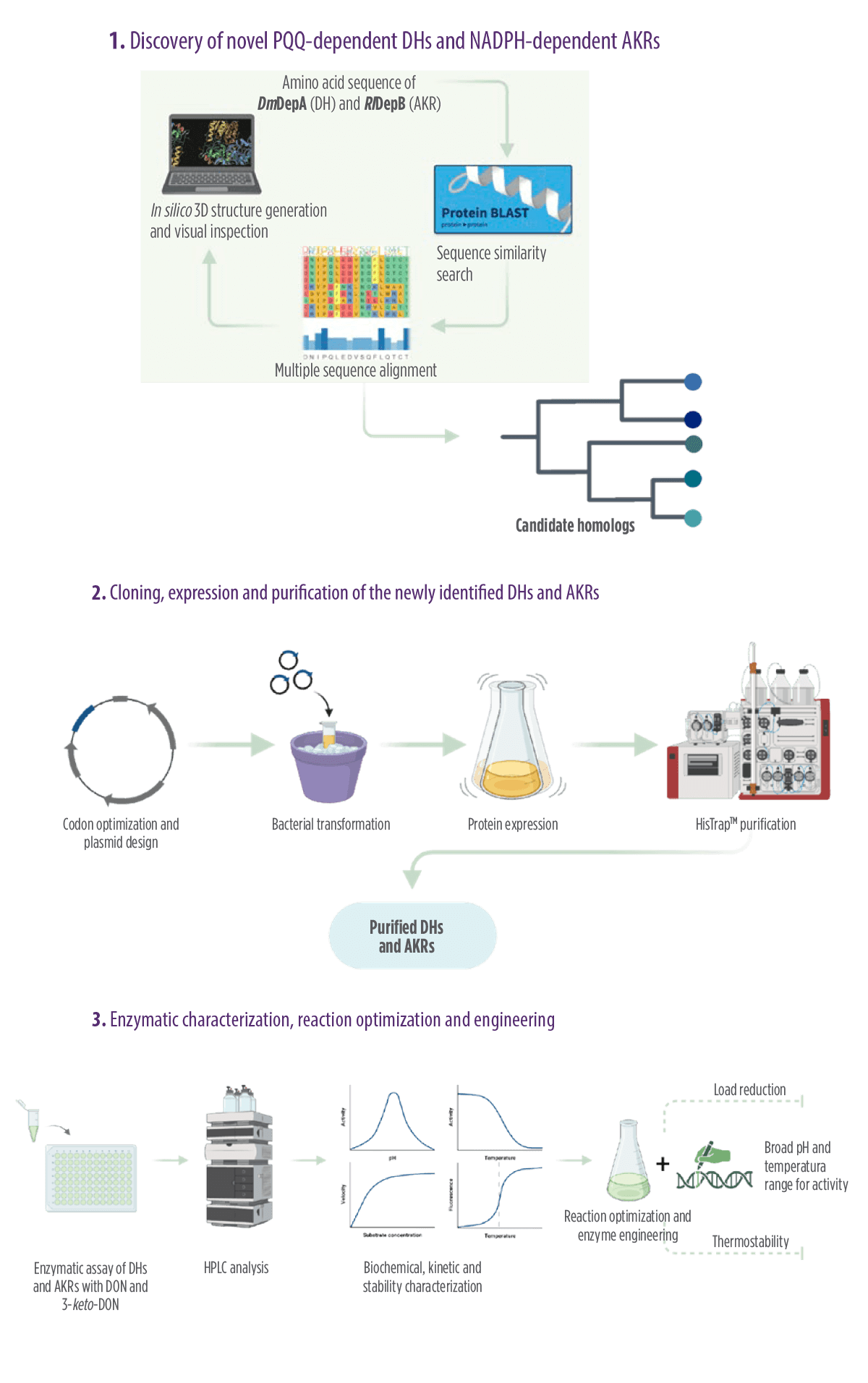
The study involved a three-step procedure:
- 1. Discovery of novel PQQ-dependent DHs and NADPH-dependent AKRs.
- 2. Cloning, expression, and purification of the newly identified DHs and AKRs.
- 3. Enzymatic characterization, reaction optimization, and engineering.

RESULTADS & CONCLUSIONS
RESULTADS & CONCLUSIONS
 As a result of this study, it was concluded that:
As a result of this study, it was concluded that:
- ⇰DON epimerization can be a solution for food and feed decontamination. However, reaction conditions need to be optimized.
- ⇰ This procedure allowed for the identification of 13 novel sequences (DHs and AKRs).
- ⇰ Analytical separation of epimers (DON and 3-epi-DON) can be challenging.
 Considering these results, in the future, it will be important to determine if:
Considering these results, in the future, it will be important to determine if:
- ⇰ The identified DHs and AKRs enzymes operate optimally under the required industrial conditions.
- ⇰ It is possible to improve stability with rational or semi-rational engineering.
Authors
Valeria Della Gala1, Jog Raj2, Hunor Farkaš2, Marko Vasiljević2, and Ditte Hededam Welner1
1TThe Novo Nordisk Foundation Center for Biosustainability, Technical University of Denmark, Kongens Lyngby, Denmark
2PATENT CO, DOO., Mišićevo, Serbia
ENZYNOV’2 Conference on Enzymatic Biocatalysis For Industry – Unleashing the power of Enzymes and Biocatalysis for industrial applications. Enzyme production challenges, Sustainability, innovations, and industrial applications. October 26-27, 2023. Biocitech, Paris-Romainville, France.
1. Kamle, M., et al. Deoxynivalenol: An Overview on Occurrence, Chemistry, Biosynthesis, Health Effects and Its Detection, Management, and Control Strategies in Food and Feed. Microbiol. Res. 13, 292-314 (2022).
2. He, J. W., et al. Toxicology of 3-epi-deoxynivalenol, a deoxynivalenol transformation product by Devosia mutans 17-2-E-8, Food and Chemical Toxicology, 84, 250-259 (2015).
3. Hassan, Y.I., et al. The enzymatic epimerization of deoxynivalenol by Devosia mutans proceeds through the formation of 3-keto-DON intermediate. Sci Rep, 7, 6929 (2017).




 Micotoxicosis prevention
Micotoxicosis prevention