S. Divyashree and M. Y. Sreenivasa
Applied mycology lab, DoS in Microbiology, University of Mysore, Mysore, India
Email: [email protected]; [email protected]
Poultry has developed into an essential economic activity and a major agricultural sector worldwide. Poultry feed is one of the most expensive items and it accounts for up to 70% of total poultry production outlay.
Microbial complications are one of the most significant aspects of poultry production, causing substantial economic losses globally.
⇰ In this article, we review several microbial complications and their current prevention strategies, mainly the use of probiotics as a next-generation therapeutic agent to combat harmful food-borne pathogens as well as their additional applications in poultry production.

Microbial infections in the poultry industry
Microbial complications caused by bacteria, viruses, fungi and their toxins have been reported worldwide as a major problem in poultry production and feed.
Bacterial contamination 
Bacterial infections are the foremost cause of food-borne gastroenteritis in humans globally due to the consumption of contaminated poultry products (Weill et al., 2006).
Contamination of poultry products with Salmonella spp., Escherichia coli, Listeria monocytogenes, Campylobacter spp. and Clostridium perfringens can be traced back to the gastrointestinal tract (caeca), where they have high activity by effectively colonizing gut epithelial cells (Pascual et al., 1999).
These microorganisms lower the body’s defense mechanisms by creating an imbalance in the intestinal microbiome, leading to the proliferation of more harmful pathogens and causing physiological disturbances such as:
 Septicemia
Septicemia
 Meningitis
Meningitis
 Encephalitis
Encephalitis
 Vomiting
Vomiting
 Diarrhea
Diarrhea
 Improper digestion
Improper digestion
 Loss of appetite and poor absorption of nutrients, which in turn, reduces immunocompetence and productivity
Loss of appetite and poor absorption of nutrients, which in turn, reduces immunocompetence and productivity
They can also lead to autoimmune disorders, kidney failure, and temporary paralysis, possibly causing permanent nerve damage and death.
In the case of the poultry industry, these infections lead to heavy economic losses (Dharma et al., 2008).
Fungal contamination 
Fungal contamination, mainly with mycotoxins, i.e., toxic secondary metabolites produced by filamentous fungi or molds such as Aspergillus spp. (aflatoxins, ochratoxins), Fusarium spp. (deoxynivalenol, fumonisins, zearalenone, and trichothecenes), and Penicillium spp. (ochratoxin A and patulin), causes high mortality and morbidity rates in poultry production.
 Due to their direct toxicity and their mutagenic, teratogenic, carcinogenic, and immunosuppressive effects, as well as their sporadic nature, they pose a significant risk in terms of detection and prediction, since they tend to accumulate in poultry feed (Hussain et al., 2010).
Due to their direct toxicity and their mutagenic, teratogenic, carcinogenic, and immunosuppressive effects, as well as their sporadic nature, they pose a significant risk in terms of detection and prediction, since they tend to accumulate in poultry feed (Hussain et al., 2010).
Viral contamination 
In addition to bacterial and fungal pathogens, gastrointestinal viral pathogens are also implicated in food-borne infections.
A wide variety of viral diseases, such as avian pox (Avipoxvirus), Newcastle disease (Paramyxovirus), infectious and quail bronchitis (influenza virus), lymphoid leukosis (Retrovirus), Marek’s disease (chicken herpes virus), and infectious bursal disease (Birnavirus) occur in poultry species.
The viruses present in respiratory secretions, feces, blood, and organs of live birds are believed to be the primary risk factor for human infections through the consumption of poultry-derived products (Rodríguez- Díaz y Monedero, 2013). Hence, the
need to control these microbial complications.
Handling the current situation of microbial complications and related problems
Antibiotic growth promoters (AGP), such as streptomycin, avoparcin, tetracycline, bacitracin, chlortetracycline, tylosin, neomycin, oxytetracycline, virginiamycin, trimethoprim/sulphonamide, lincosamides, cephalosporins, etc. have been used in poultry production for the last five decades to control microbial diseases.
However, antibiotics and hormonal growth promoters in poultry production have been widely banned by the European Union since 2006 (Jha et al., 2020) to the growing evidence of transfer of antibiotic resistance genes and allergies from farm animals to humans (Vieco-Saiz et al., 2019).
AGPs have also been reported to alter the gut microbiome and, in consequence, the modulation of the immune system, as well as negatively affecting weight gain, substantially decreasing production. However, the elimination of AGPs has increased the incidence of animal diseases (Bajagai et al., 2016; Jha et al., 2019).
Many feed additives are known for their antimicrobial properties. Therefore, phytobiotics (plant-based feed additives), organic acids, chitin, medium (caproic acid, capric acid, lauric acid, and caprylic acid) and long-chain fatty acids (polyunsaturated fatty acids), prebiotics, enzymes, antimicrobial peptides, hyperimmune IgY, bacteriophages, and clay have been used as alternatives to replicate the effects of AGPs.
Their positive effects include:
 Prevention of microbial diseases
Prevention of microbial diseases
 Immunomodulation
Immunomodulation
 Improvement of the quality of poultry products
Improvement of the quality of poultry products
 Feed intake (FI) and feed conversion ratio (FCR) improvement
Feed intake (FI) and feed conversion ratio (FCR) improvement
However, these feed additives have several disadvantages, for example:
 Phytobiotics have toxic parts (Ogbuewu et al., 2020).
Phytobiotics have toxic parts (Ogbuewu et al., 2020).
 Organic acids have shown instability, corrosivity, and unpleasant smell, and can alter the lactic acid bacteria present in the gastrointestinal tract (El-Hack et al., 2020).
Organic acids have shown instability, corrosivity, and unpleasant smell, and can alter the lactic acid bacteria present in the gastrointestinal tract (El-Hack et al., 2020).
 Khempaka et al. (2006) reported the reduction of digestibility and growth performance in chickens due to the use of chitin as feed additives.
Khempaka et al. (2006) reported the reduction of digestibility and growth performance in chickens due to the use of chitin as feed additives.
 Medium and long-chain fatty acids cause coronary heart diseases and depression problems (Thapa, 2020).
Medium and long-chain fatty acids cause coronary heart diseases and depression problems (Thapa, 2020).
 Surfactants have bactericidal and bacteriostatic effects, but they can cause environmental pollution leaving traces in terrestrial and aquatic environments (Viegas y Sa-Correia, 1991).
Surfactants have bactericidal and bacteriostatic effects, but they can cause environmental pollution leaving traces in terrestrial and aquatic environments (Viegas y Sa-Correia, 1991).
 Other feed additives are dose-dependent and show adverse side effects.
Other feed additives are dose-dependent and show adverse side effects.

Figure 1. Different feed additives used in poultry production.
The most commonly employed detoxification method in the poultry industry is the use of multi-spectrum mycotoxin binders (sequestering agents) in feed.
⇰ It is common to use a combination of Picrorhiza kurroa, activated charcoal, hydrated sodium calcium aluminosilicate (HSCAS), mannan oligosaccharide (MOS), buffered organic acids, and antioxidants. However, these compounds have less affinity towards mycotoxins (Huang et al., 2019).
 Considering all these drawbacks, alternative biocontrol probiotic agents have shown their versatility and compatibility to decrease the problems of bacterial resistance to antibiotics, prevent and control diseases, decrease mortality rates, and enhance the growth of farm animals. Hence, they may be useful in the poultry industry.
Considering all these drawbacks, alternative biocontrol probiotic agents have shown their versatility and compatibility to decrease the problems of bacterial resistance to antibiotics, prevent and control diseases, decrease mortality rates, and enhance the growth of farm animals. Hence, they may be useful in the poultry industry.
Probiotic-based therapy against microbial complications
The term “Probiotic” is a Greek word that means “pro-life”. Probiotics have been gaining more therapeutic relevance over the years with increasing scientific knowledge about their association with intestinal health and immunity, leading to the overall well-being of animals/humans. They usually have a natural origin, coming from fermented food products or plant products and/ or from the intestinal tract of animals and humans (Clarke et al., 2012).

 FAO/WHO (Food and Agricultural Organization/World Health Organization, 2001) defined these beneficial organisms as “Live microbes when administered in proper amounts beneficially affect the health of the host”.
FAO/WHO (Food and Agricultural Organization/World Health Organization, 2001) defined these beneficial organisms as “Live microbes when administered in proper amounts beneficially affect the health of the host”.
Potential probiotic organisms are non-pathogenic and are generally recognized as safe (GRAS). They are capable of resisting the effects of gastric/intestinal juices, showing:
 An antibiotic resistance profile
An antibiotic resistance profile
 Tolerance to bile
Tolerance to bile
 Adhesion to intestinal epithelial cells
Adhesion to intestinal epithelial cells
 Production of antimicrobial substances such, as bacteriocins, organic acids, esters, fatty acids, diacetyl, reuterin, reutericyclin and hydrogen peroxide that exhibits antimicrobial properties
Production of antimicrobial substances such, as bacteriocins, organic acids, esters, fatty acids, diacetyl, reuterin, reutericyclin and hydrogen peroxide that exhibits antimicrobial properties
(Coman et al., 2014)
In addition, antagonism is one of the most important probiotic control strategies based on two basic mechanisms:
1. Competitive exclusion:
 Competition for nutrients
Competition for nutrients
 Production of antimicrobial compounds
Production of antimicrobial compounds
 Prevention of the adhesion of pathogens to epithelial cells
Prevention of the adhesion of pathogens to epithelial cells
 Reduction of toxin bioavailability
Reduction of toxin bioavailability
2. Immune modulation that involves the stimulation of host defense systems (Solis-cruz et al., 2018; Patlan et al., 2019).
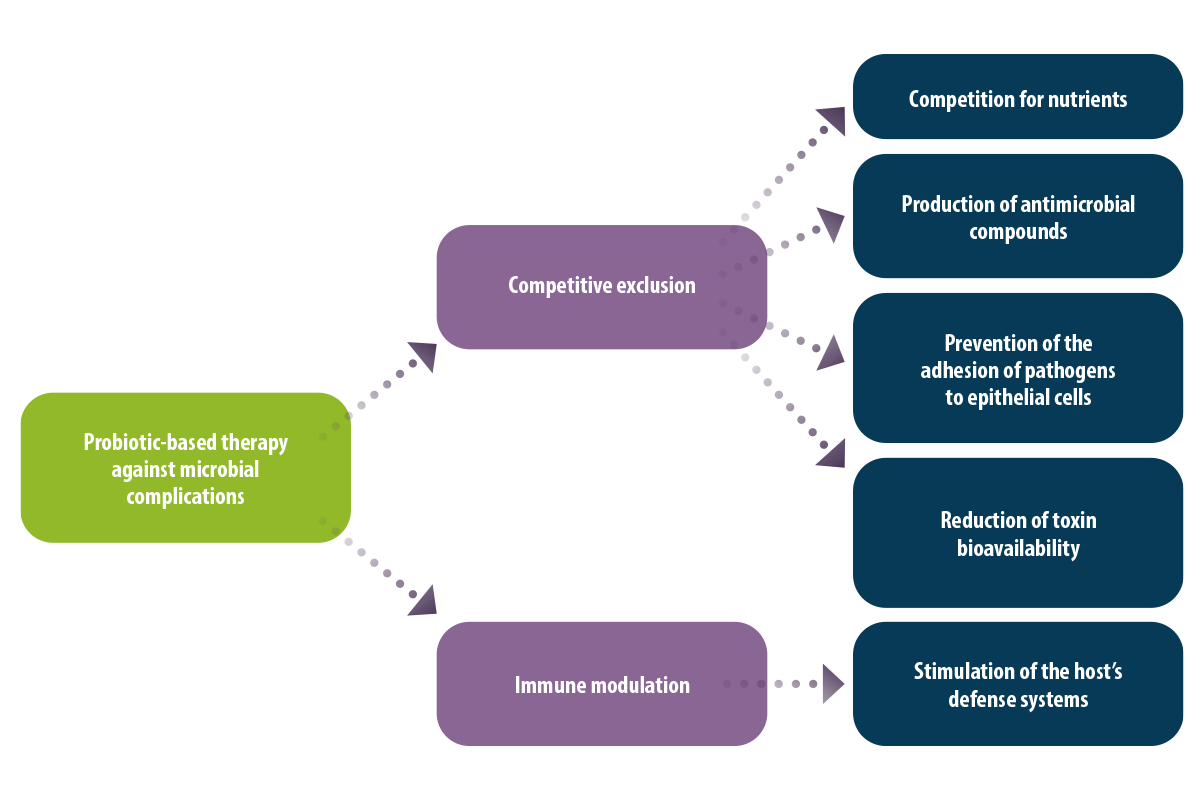
Figure 2. Probiotic-based therapy to combat harmful pathogens in the poultry industry.
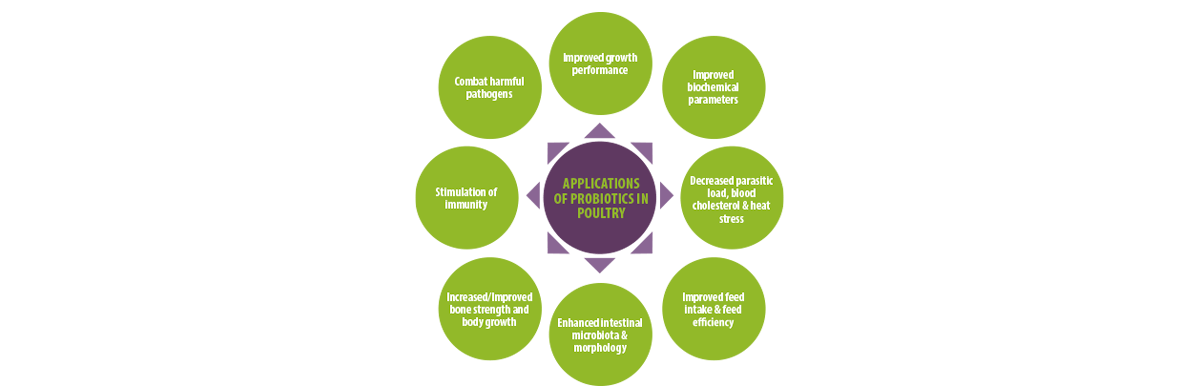
Figure 3. The applications of probiotics in poultry production.
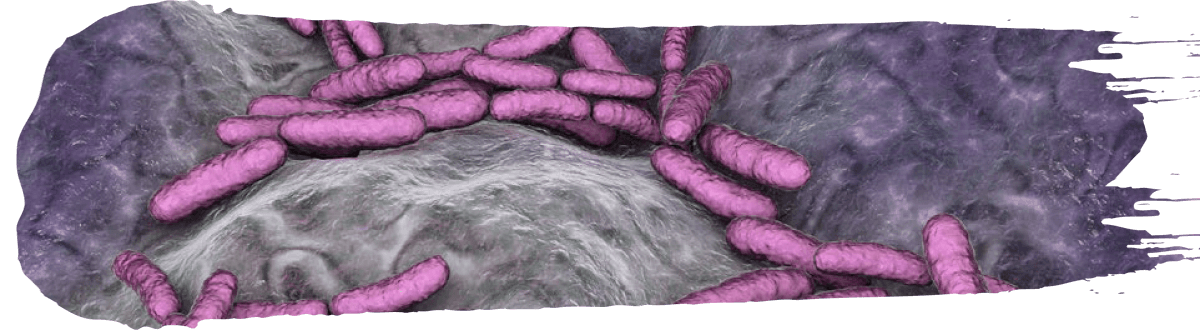
Probiotics against harmful bacteria pathogens 
Probiotics have been extensively tested and validated against bacterial pathogens.
Numerous articles have been published on the use of probiotics as an antibiotic replacement by reducing the growth of pathogens such as Salmonella paratyphi (Divyashree et al., 2021), E. coli (Tsai et al., 2010), Listeria monocytogenes (Gil De Los Santos et al., 2012) and Clostridium perfringens (Santos, 2012) in in vitro and in vivo models. For example:

Saccharomyces cerevisiae, at 0.4% and 0.8% reduced the gastrointestinal microbial load of E. coli, Micrococcus spp., Klebsiella spp., Staphylococcus spp., Campylobacter spp, and Clostridium perfringens in layers (Hassanein et al., 2010).
Broiler chickens orally administered with Lactobacillus salivarius showed effective prevention of Campylobacter jejuni intestinal colonization (Saint-Cyr et al., 2017). In another study, feeding bacteriocins (250mg) from Lactobacillus salivarius and Paenibacillus polymyxa showed a reduction in C. jejuni colonization (Timbermont et al., 2010).

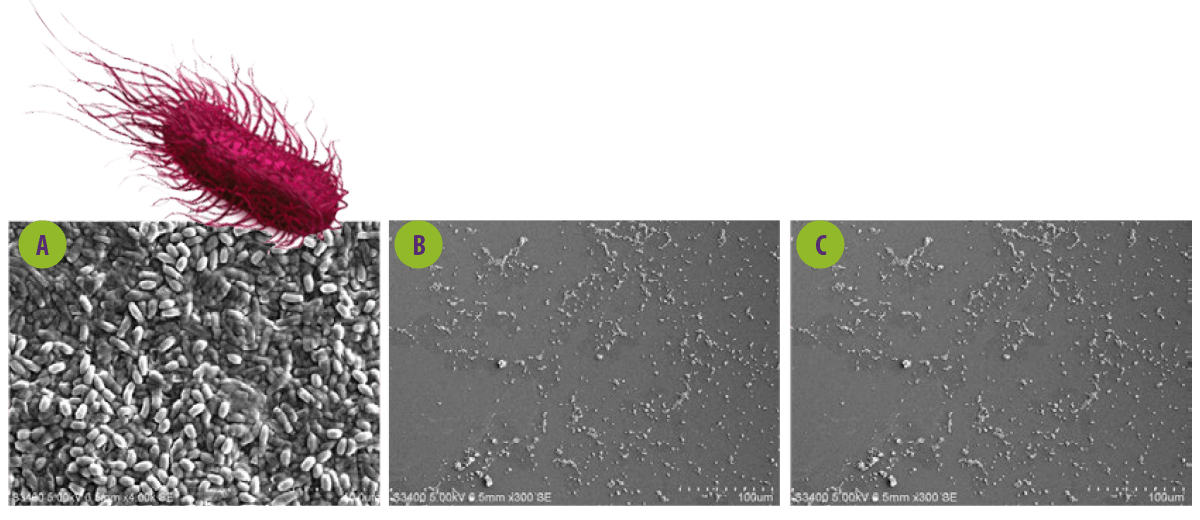
Figure 4. Scanning electron microscopy of the effect of cell-free supernantant from probiotics on Salmonella biofilms. A. SEM of Salmonella paratyphi ATCC 9150. B & C. S.paratyphi treated with Lactobacillus spp.
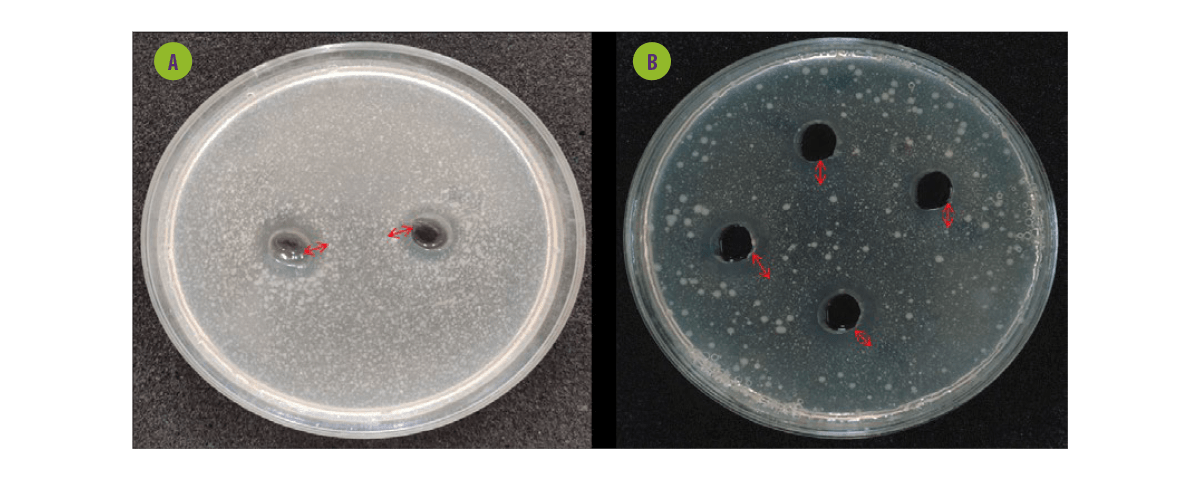
Figure 5. Agar well diffusion assay of probiotics against bacteria food-borne pathogens. A. Cell-free supernatant from probiotics on Escherichia coli. B. Cell-free supernatant from probiotics on Salmonella paratyphi.
Probiotics against fungi and their mycotoxins 
Probiotics are well known for their antifungal activity and for inactivating mycotoxins in poultry.
![]() A study conducted by Deepthi et al. (2017), reported a significant role of L. plantarum MYS6 in ameliorating the FB1-induced toxicity (fumonisin produced by Fusarium verticilliodes) in the vital organs and subsequent oxidative stress in broilers.
A study conducted by Deepthi et al. (2017), reported a significant role of L. plantarum MYS6 in ameliorating the FB1-induced toxicity (fumonisin produced by Fusarium verticilliodes) in the vital organs and subsequent oxidative stress in broilers.
![]() Lacticaseibacillus brevis MYSN105 and L. plantarum MYS6 showed potential antagonistic activity by showing morphological alterations such as damaged mycelia and deformed conidia against fumonisin producing F. verticillioides and F. proliferatum, respectively, in in vitro assays (Somashekaraiah et al., 2021; Deepthi et al., 2016).
Lacticaseibacillus brevis MYSN105 and L. plantarum MYS6 showed potential antagonistic activity by showing morphological alterations such as damaged mycelia and deformed conidia against fumonisin producing F. verticillioides and F. proliferatum, respectively, in in vitro assays (Somashekaraiah et al., 2021; Deepthi et al., 2016).
![]() The cell-free supernatant (CFS) from Lactobacillus plantarum strain MYS44 was suggested as a potential detoxification agent due to its antifungal activity and its capacity to reduce aflatoxin B1 (34.2%) produced by Aspergillus parasiticus MTCC 411 (Rao et al., 2017).
The cell-free supernatant (CFS) from Lactobacillus plantarum strain MYS44 was suggested as a potential detoxification agent due to its antifungal activity and its capacity to reduce aflatoxin B1 (34.2%) produced by Aspergillus parasiticus MTCC 411 (Rao et al., 2017).

Figure 6. Use of probiotics against Aspergillus species.
A. Antifungal activity of Lactobacillus against Aspergillus flavus.
B. Antifungal activity of Lactobacillus against Aspergillus parasiticus.

Figure 7. Use of probiotics against Fusarium species. The antifungal activity of the LAB isolates was tested against F. sporotrichioides (1), F. equiseti (2), F poea (3), F. avenaceum (4), F. verticillioides (5), F. graminearum (6), A. parasiticus (7), and Rhizopus oryzae (8).
A. Fungal control plates after 7 days of incubation.
B. Antifungal activity of isolate Lactobacillus plantarum MYSRD 71 after 7 days of incubation against all the fungal species tested.
Several studies have been conducted to evaluate the mycotoxin neutralization capacity of several probiotic strains such as Propionibacterium, L. rhamnosus GG, L. plantarum, L. casei, Bacillus species, and E. faecium (Danicke and Doll, 2010).
⇰ For example, Saccharomyces cerevisiae could adsorb zearalenone and ochratoxin under simulated gastrointestinal conditions in a mycotoxin binding assay (Armando et al., 2012).
In studies conducted by Patlan et al. (2019), probiotic Bacillus spp. were able to biodegrade aflatoxin B1 in both in vitro and in vivo models of broiler chickens.
In another study, L. casei and L. reuteri reduced oxidative stress induced by aflatoxins in mice (Hernández-Mendoza et al., 2011).
Probiotics for the control of viral pathogens 
Probiotics have also been used to control viruses in poultry.
![]() Administration of L. paracasei protected against rotavirus-induced diarrhea (Pant et al., 2006), while a study conducted by Wen et al. (2009) with L. acidophilus and L. reuteri decreased rotavirus infections by enhancing IFN- ץ and IL-4 in serum.
Administration of L. paracasei protected against rotavirus-induced diarrhea (Pant et al., 2006), while a study conducted by Wen et al. (2009) with L. acidophilus and L. reuteri decreased rotavirus infections by enhancing IFN- ץ and IL-4 in serum.
![]() Naseem et al. (2012) reported that broilers fed with a Lactobacillus protein supplemented diets had increased antibody titers compared with the controls against infectious bursal disease, influenza disease, and Newcastle disease virus.
Naseem et al. (2012) reported that broilers fed with a Lactobacillus protein supplemented diets had increased antibody titers compared with the controls against infectious bursal disease, influenza disease, and Newcastle disease virus.
Additional applications of probiotics in poultry
Probiotic effects on growth performance 
In poultry production, several strategies are used to improve the number of eggs laid, increase the egg weight, and improve the eggshell quality, egg yolk cholesterol, albumin quality, and meat quality.
The administration of probiotics with hens’ diets improves laying production by increasing nitrogen and calcium retentions, as well as daily feed consumption.
⇰ It has been proposed that probiotics increase the production of short-chain fatty acids (SCFA) and the intestinal fermentation rate which provides nourishment for intestinal epithelial cells, which also leads to improved mineral assimilation (Alagawany et al., 2018).
A study conducted by Abd EI-Hack et al. (2017) showed that supplementation with multi-strain probiotics increased egg production, including albumin and yolk weight, egg weight and size, and the eggshell strength and thickness when compared to the control group.
Decreased parasitic load, blood cholesterol and significant effect on heat stress 
 Recently, researchers have shown that dietary supplementation of probiotics decreases the level of cholesterol, very-low-density lipoproteins (VLDL), and triglycerides in the blood (Kanani et al., 2018; Shirley et al., 2017).
Recently, researchers have shown that dietary supplementation of probiotics decreases the level of cholesterol, very-low-density lipoproteins (VLDL), and triglycerides in the blood (Kanani et al., 2018; Shirley et al., 2017).
 Lee et al. (2007) demonstrated that the supplementation of probiotics enhances the resistance against coccidiosis (growth depression) and reduces the level of Eimeria tenella and E. acervulina infection in poultry.
Lee et al. (2007) demonstrated that the supplementation of probiotics enhances the resistance against coccidiosis (growth depression) and reduces the level of Eimeria tenella and E. acervulina infection in poultry.
 Heat stress increases pathogen colonization, and immunosuppression, promoting the onset of infections (Ursell et al., 2012). However, feeding probiotics to broilers has been shown to mitigate the impact of heat stress by maintaining a healthy gut microbiota in stressful environmental conditions and increasing the length of villi.
Heat stress increases pathogen colonization, and immunosuppression, promoting the onset of infections (Ursell et al., 2012). However, feeding probiotics to broilers has been shown to mitigate the impact of heat stress by maintaining a healthy gut microbiota in stressful environmental conditions and increasing the length of villi.
Stimulation of immunity and improvement of biochemical parameters 
Pathogens must overcome obstacles (the physical barrier of the epithelium, the inhibitory effects of the gut microbiota, and the host’s immune system response) to colonize the intestinal tract and successfully cause an infection. However, probiotics are well known for the modulation of intestinal environments.
![]() They bind to the Toll-like receptors of sentinel cells (epithelial cells and dendritic cells of the intestine) and activate the NF-κB and MAP kinase pathways (Bai et al., 2013).
They bind to the Toll-like receptors of sentinel cells (epithelial cells and dendritic cells of the intestine) and activate the NF-κB and MAP kinase pathways (Bai et al., 2013).
⇰ This activation causes the suppression or upregulation of genes that regulate antigen presentation, as well as cytoprotective effects through immune activation, inflammatory response, and the expression of antimicrobial factors (Kemgang et al., 2014).
![]() Their benefits include increased adhesion of beneficial bacteria to the intestinal mucosa, improved epithelial barrier, and the inhibition of pathogen adhesion (Broom y Kogut, 2018).
Their benefits include increased adhesion of beneficial bacteria to the intestinal mucosa, improved epithelial barrier, and the inhibition of pathogen adhesion (Broom y Kogut, 2018).
Numerous studies have shown the impact of probiotics on biochemical parameters, for example, improvement in the levels of blood glucose, omega 6 and essential polyunsaturated fatty acids (linoleic acid and linolenic acid) and propionate (SCFA) in egg yolk (Tang et al., 2016; Ejtahed et al., 2012; Trautwein et al., 1998).
Improved bone strength and increase in body growth, feed intake, and feed efficiency 
Probiotics modulate the gut environment, enhance the barrier function, and promote the growth of poultry animals (Deepthi et al., 2017). By colonizing the intestine, they compete with pathogens for nutrients and prevent their establishment. However, these increase body weight, feed intake, and feed efficiency (Jadhav et al., 2015).

In addition, probiotic supplementation in poultry feed also improved the thickness of the medial and lateral wall of the tibia and tibiotarsal index, which are indicators of bone strength.
Influence of probiotics on the intestinal microbiota and morphology 
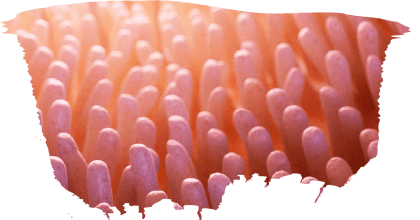
Supplementation of probiotics for dietary treatment has been shown to influence intestinal villi length and decrease crypt depth by enhancing gut microbiota development for the control of pathogens population.
⇰ Therefore, crypts in enterocytes replace the cells that have been lost due to inflammation at the villus tip by continuous proliferation (Cox y Dalloul.2009: Awad et al., 2009).
CONCLUSIONS

REFERENCES
Abd El-Hack, M.E.; Mahgoub, S.A.; Alagawany, M.; Ashour, E.A., 2017. Improving productive performance and mitigating harmful emissions from laying hen excreta via feeding on graded levels of corn DDGS with or without Bacillus subtilis probiotic. J. Anim. Physiol. Anim. Nutr. 101, 904–913.
Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Sachan, S.; Karthik, K.; Dhama, K.,2018. The use of probiotics as eco-friendly alternatives for antibiotics in poultry nutrition. Environ. Sci. Pollut. Res. 25, 10611–10618.
Angel, R.; Dalloul, R.A. & Doerr, J., 2005. Performance of broilers chickens fed diets supplemented with a direct-fed microbial. Poultry Science, Vol.84, (August 2005), pp. 1222-1231, ISSN 1525-3171.
Armando, M. R.; Pizzolitto, R. P.; Dogi, C. A.; Cristofolini, A.; Merkis, C,; Poloni, V. L., et al. 2012. Adsorption of ochratoxin A and zearalenone by potential probiotic Saccharomyces cerevisiae strains and its relation with cell wall thickness. Journal of Applied Microbiology, 113, 256–264.
Awad, W. S; Ghareeb and J. Bohm, 2009. Probiotic and symbiotic on growth performance, organ weights and intestinal histomorphology of broiler chickens. Poultry Science, 88: 49-56.
Bajagai, Y.S.; Klieve, A.V.; Dart, P.J.; Bryden, W.L.,2016. Animal Production and Health Div Probiotics. In Animal Nutrition: Production, Impact and Regulation; Food and Agriculture Organization of the United Nations: Rome, Italy; ISBN 978-92-5-109333-7.
Bai, S.P.; Wu, A.M.; Ding, X.M.; Lei, Y.; Bai, J.; Zhang, K.Y.; Chio, J.S.,2013. Effects of probiotic-supplemented diets on growth performance and intestinal immune characteristics of broiler chickens. Poult. Sci. 92, 663–670
Clarke G.; Cryan, J.F.; Dinan, T.G.; et al. 2012. Review article: probiotics for the treatment of irritable bowel syndrome: focus on lactic acid bacteria. Aliment Pharmacol Ther 35(4):403–413
Coman, M. M.; Verdenelli, M. C.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Boyko, N.; & Cresci, A., 2014. In vitro evaluation of antimicrobial activity of Lactobacillus rhamnosus IMC 501®, Lactobacillus paracasei IMC 502® and SYNBIO® against pathogens. Journal of Applied Microbiology, 117(2), 518–527. doi:10.1111/jam.12544
Cox, C.M.; and R. Dalloul,, 2009. Immuno-modulatory role of probiotics in poultry and potential in ova application. Beneficial microbes, 61: 45-52
Danicke, S.; & Doll, S., 2010. A probiotic feed additive containing spores of Bacillus subtilis and B. licheniformis does not prevent absorption and toxic effects of the Fusarium toxin deoxynivalenol in piglets. Food and Chemical Toxicology, 48, 152–158
Deepthi, B.V.; Poornachandra Rao, K.; Chennapa, G.; Naik, M.K.; Chandrashekara, K.T.; Sreenivasa, M.Y., 2016 Antifungal Attributes of Lactobacillus plantarum MYS6 against Fumonisin Producing Fusarium proliferatum Associated with Poultry Feeds. PLoS ONE 11(6): e0155122. https://doi.org/10.1371/journal.pone.0155122
Deepthi, B. V.; Somashekaraiah, Rakesh; Poornachandra Rao, K.; Deepa, N.; Dharanesha, N. K.; Girish, K. S.; Sreenivasa, M. Y. 2017. Lactobacillus plantarum MYS6 Ameliorates Fumonisin B1-Induced Hepatorenal Damage in Broilers. Frontiers in Microbiology, 8, 2317–. doi:10.3389/fmicb.2017.02317
Dhama, K.; Mahendran, M.; Simmi Tomar,; and Chauhan, R. S., 2008. Beneficial effects of probiotics and prebiotics in livestock and poultry: the current perspectives. Intas Polivet, 9(1): 1-13.
Huang, W.; Chang, J.; Wang, P.; Liu, C.; Yin, Q.; Song, A.; Gao, T.; Dang, X.; & Lu, F. ,2019. Effect of Compound Probiotics and Mycotoxin Degradation Enzymes on Alleviating Cytotoxicity of Swine Jejunal Epithelial Cells Induced by Aflatoxin B₁ and Zearalenone. Toxins, 11(1), 12. https://doi.org/10.3390/toxins11010012
Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; and Mofid, V., 2012. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 28, 539-543.
El-Hack, M.A.; Shafi, M.; Alghamdi, W.; Abdelnour, S.; Shehata, A.; Noreldin, A.; Ashour, E.; Swelum, A.; Al-Sagan, A.; Alkhateeb, M. et al. 2020. Black Soldier Fly (Hermetia illucens) Meal as a Promising Feed Ingredient for Poultry: A Comprehensive Review. Agriculture 10, 339.
Gil de los Santos, J. R.; Storch, O. B.; Fernandes, C. G., & Gil-Turnes, C., 2012. Evaluation in broilers of the probiotic properties of Pichia pastoris and a recombinant P. pastoris containing the Clostridium perfringens alpha toxin gene. Veterinary Microbiology, 156, 448–451.
Hassanein, S.M.; Soliman, N.K., 2010. Effect of Probiotic (Saccharomyces cerevisiae) Adding to Diets on Intestinal Microflora and Performance of Hy-Line Layers Hens. Journal of American Science. 6:159-169
Hernandez-Mendoza, A.; Gonza´lez-Co´rdova, A. F.; Vallejo-Cordoba, B.; & Garcia, H. S., 2011. Effect of oral supplementation of Lactobacillus reuteri in reduction of intestinal absorption of aflatoxin B1 in rats. Journal of Basic Microbiology, 51, 263–268.
Hussain, Z.; Muhammad Zargham Khan, Ahrar Khan; Ijaz Javed; Muhammad Kashif Saleemi; Sultan Mahmood; Muhammad Rafique Asi., 2010. Residues of aflatoxin B1 in broiler meat: Effect of age and dietary aflatoxin B1 levels. , 48(12), 0–3307. doi:10.1016/j.fct.2010.08.016
Jadhav, K.; Katoch, S.; Sharma, V.K.; Mane, B.G., 2015. Probiotics in Broiler Poultry Feeds: A Review. J. Anim. Nutr. Physiol. 1, 4–16
Jha, R.; Fouhse, J.M.; Tiwari, U.P.; Li, L., 2019. Willing, B.P. Dietary Fiber and Intestinal Health of Monogastric Animals. Front. Vet. Sci. 6, 48.
Jha, R.; Razib Das,; Sophia Oak and Pravin Mishra., 2020. Probiotics (Direct-Fed Microbials) in Poultry Nutrition and Their Effects on Nutrient Utilization, Growth and Laying Performance, and Gut Health: A Systematic Review. Animals 10, 1863; doi: 10.3390/ani10101863.
Kanani, P.B.; Hosseintabar, B. G.; Youvalari, S.A.; Seidavi, A.; Ragni, M.; Laudadio, V., and Tufarelli, V., 2018. Effects of using artemisia annua leaves, probiotic blend, and organic acids on performance, egg quality, blood biochemistry, and antioxidant status of laying hens. The Journal of Poultry Science 56:120-127.
Kemgang, T.S.; Kapila, S.; Shanmugam, V.P.; Kapila, R., 2014. Cross-talk between probiotic lactobacilli and host immune system. J. Appl. Microbiol. 117, 303–319
Khempaka, S.; Mochizuki, M.; Koh, K.; Karasawa, Y., 2006. Effect of Chitin in Shrimp Meal on Growth Performance and Digestibility in Growing Broilers. J. Poult. Sci. 43, 339–343.
Lee, S.H.; Lillehoj, H.S.; Dalloul, R.A.; Park, D.W.; Hong, Y.H.; Lin, J.J., 2007. Influence of pediococcus-based probiotic on coccidiosis in broiler chickens. Poultry Science. 86:63-66.
Naseem, S.; Rahman, S.U.; Shafee, M.; Sheikh, A.A.; Khan. A..2012. Immunomodulatory and growth-promoting effect of a probiotic supplemented in the feed of broiler chicks vaccinated against infectious bursal disease. Brazilian Journal of Poultry Science. 14:109-113.
Ogbuewu, I.; Okoro, V.; Mbajiorgu, C., 2020. Meta-analysis of the influence of phytobiotic (pepper) supplementation in broiler chicken performance. Trop. Anim. Health Prod. 52, 17–30.
Pant, N.; Hultberg, A.; Zhao, Y. F.; Svensson, L.; Pan-Hammarstrom, Q.; Johansen, K., et al. 2006. Lactobacilli expressing variable domain of llama heavy-chain antibody fragments (lactobodies) confer protection against rotavirus-induced diarrhea. The Journal of Infectious Diseases, 194, 1580–1588.
Pascual, M.; Hugas, M.; Badiola, J.I.; Monfort, J.M.; Garriga, M., 1999. Lactobacillus salivarius CTC2197 prevents Salmonella enteritidis colonization in chickens. Applied Environmental Microbiology. 65:4981-4986
Patlan, D.H.; Bruno Solis-Cruz,; Billy M. Hargis and Guillermo Tellez., 2019. The Use of Probiotics in Poultry Production for the Control of Bacterial Infections and Aflatoxins. DOI: org/10.5772/intechopen.88817.
Poornachandra Rao, K.; Deepthi, B. V.; Rakesh, S.; Ganesh, T.; Achar, Premila; Sreenivasa, M. Y. 2017. Antiaflatoxigenic Potential of Cell Free Supernatant from Lactobacillus plantarum MYS44 Against Aspergillus parasiticus. Probiotics and Antimicrobial Proteins, doi:10.1007/s12602-017-9338-y
Rakesh, Somashekaraiah.; Walid, M.; Adithi, G.; Udith, J.; Riad, H.; and M Y Sreenivasa. 2021. Probiotic and Antifungal Attributes of Levilactobacillus brevis MYSN105, Isolated From an Indian Traditional Fermented Food Pozha. Frontiers in Microbiology. 12:696267. doi: 10.3389/fmicb.2021.696267,
Rodriguez-Diaz, J.; and V. Monedero., 2013. Probiotics against digestive tract viral infections. In Bioactive food as detary interventions for liver and gastrointestinal disease, ed. V. R. Preedy. San Diego, Academic Press, 271–84
Saint-Cyr.; Manuel Jimmy.; Haddad, Nabila.; Taminiau, Bernard.; Poezevara, Typhaine.; Quesne, Ségolène.; Amelot, Michel.; Daube, Georges Chemaly, Marianne.; Dousset, Xavier.; Guyard-Nicodème, Muriel., 2016. Use of the potential probiotic strain Lactobacillus salivarius SMXD51 to control Campylobacter jejuni in broilers. International Journal of Food Microbiology, S016816051630349X–. doi:10.1016/j.ijfoodmicro.2016.07.003
Shirley, G.H.T.; Chin, C.S.; Kalavathy, R.; Wan, Z. S.; Hee, K.W.; and Yin,W.; 2017. Performance, biochemical and haematological responses, and relative organ weights of laying hens fed diets supplemented with prebiotic, probiotic and Symbiotic. BMC Veterinary Research 13:2-12.
Solis-Cruz, B.; Hernandez-Patlan, D.; Hargis, B.; Téllez G., 2018. Control of Aflatoxicosis in Poultry Using Probiotics and Polymers [Online First]. In: Micotoxins-Impact and management strategies. IntechOpen; DOI: 10.5772/intechopen.76371. Available from: https://www.intechopen.com
Tang, C.; Hoo, P.C.; Tan, L.T.; Pusparajah, P.; Khan, T.M.; Lee, L.H.; Goh, B.H., and Chan, K.G., 2016. Golden needle mushroom: A culinary medicine with evidenced-based biological activities and health promoting properties. Front. Pharmacol. 7, 474- 482.
Thapa, P., 2020. Application of micro algae in poultry nutrition: A review. J. Agric. Nat. Resour.
Timmerman, H.M.; Veldman, A.; Van Den Elsen, E.; Rombouts, F.M., Beynen, A.C., 2006. Mortality and growth performance of broilers given drinking water supplemented with chicken specific probiotics. Poultry Science. 85(8):1383-1388.
Trautwein, E.A.; Rieckhoff, D. and Erbersdobler, H. F., 1998. Dietary inulin lowers plasma cholesterol and triacylglycerol and alters biliary bile acid profile in hamsters. J. Nutr. 128, 1937-1943
Tsai, Y. T.; Cheng, P. C., & Pan, T. M., 2010. Immunomodulating activity of Lactobacillus paracasei subsp. paracasei NTU 101 in enterohemorrhagic Escherichia coli O157H7- infected mice. Journal of Agricultural and Food Chemistry, 58, 11265–11272.
Ursell, L. K.; Clemente, J. C.; Rideout, J. R.; Gevers, D.; Caporaso, J. G., & Knight, R., 2012. The interpersonal and intrapersonal diversity of human-associated microbiota in key body sites. The Journal of Allergy and Clinical Immunology, 129, 1204–1208. https://doi.org/10.1016/j.jaci.2012.03.010
Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I., & Drider, D., 2019. Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Frontiers in Microbiology, 10, 57.
Viegas, C.A.; Sa-Correia, I., 1991. Activation of plasma membrane ATPase of Saccharomyces cerevisiae by octanoic acid. J. Gen. Microbiol. 137, 645–651
Weill, F X.; Guesnier, F.; Guibert, V.; Timinouni, M.; Demartin, M.; Polomack, L., et al., 2006. Multidrug resistance in Salmonella enterica serotype Typhimurium from humans in France (1993 to 2003). Journal of Clinical Microbiology, 44, 700–708
Wen, K.; Azevedo, M. S. P.; Gonzalez, A.; Zhang, W.; Saif, L. J.; Li, G. H., et al., 2009. Toll-like receptor and innate cytokine responses induced by lactobacilli colonization and human rotavirus infection in gnotobiotic pigs. Veterinary Immunology and Immunopathology, 127, 304–315




 Micotoxicosis prevention
Micotoxicosis prevention