B. Shruthi and Sreenivasa M Y*
Applied Mycology Lab, Department of Studies in Microbiology, University of Mysore, Mysuru-570006, Karnataka, India.
*Corresponding author
E-mail: [email protected]; [email protected]
The poultry industry is a rapidly growing sector around the world, especially in India with approximately an 8% growth rate per year (Chatterjee and Rajkumar, 2015).
 Poultry feed represents a key expense in poultry production, accounting for up to 70% of the total cost.
Poultry feed represents a key expense in poultry production, accounting for up to 70% of the total cost.
Approximately 95% is dedicated to meet energy, strength, and protein requirements, around 3-4% supports important nutrient, micronutrient and vitamin needs, and 1-2% is composed of various feed additives.
Poultry feeds are formulated from a blend of constituents, including cereal grains, vitamins, cereal by-products, animal byproducts, fats, nutrient supplements, amino acids, plant protein sources and, feed additives (Ravindran, 2013).

 The Food and Drug Organization (FAO) calculates that more than 25% of the food manufactured worldwide is contaminated with fungi and their mycotoxins to a certain degree (Sreenivasa et al., 2008; 2011 and Deepa et al., 2016).
The Food and Drug Organization (FAO) calculates that more than 25% of the food manufactured worldwide is contaminated with fungi and their mycotoxins to a certain degree (Sreenivasa et al., 2008; 2011 and Deepa et al., 2016).
Current investigations have been conducted to assess the frequency of mycotoxin contamination and, globally, 30% of food and feedstuffs are co-contaminated (Magnoli et al., 2019).
Mycotoxins are toxins naturally produced by some fungal species, such as Aspergillus, Fusarium, Penicillium, Claviceps, and Alternaria. Up to date, approximately 10,000 fungi have been identified and over 500 mycotoxins have been described as potentially harmful.

The most prominent mycotoxins influencing anthropomorphs include aflatoxins (AF), fumonisins (FUM), ochratoxins (OT), patulin (PAT), trichothecenes (TCT), citrinin (CT), zearalenone (ZEN), and alkaloids (EA) (Haque et al., 2020; Deepa et al., 2021; Adithi et al., 2022).
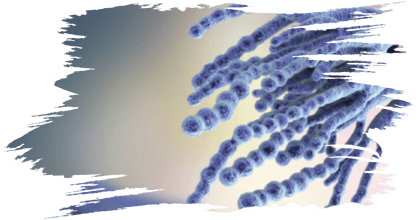
These toxins have received a great deal of attention because of their potential detrimental effect on living beings. Foods, feeds, and cereal grains are known to become contaminate with many different fungi and their toxins (Figure 1) (Sreenivasa et al., 2008; Deepa and Sreenivasa, 2018; Nagaraja et al., 2016).

Figure 1. Various mycotoxigenic fungi contaminating poultry feed. A. Feed without contamination. B and C. Growth of numerous fungi on poultry feed when supplied with moisture and incubated in dark.
Increasing consciousness towards conservation, protection, and food safety has given rise to the use of ecological and biosafe approaches for mycotoxin eradication in feed and food production (Luo t al., 2020).
Recently, biological approaches have shown great potential, with maximum efficacy without any impact on product quality.
The use of yeasts in poultry production
Yeasts are single-celled eukaryotic microorganisms with 3 – 4 μm in diameter (Walker et al., 2002). Among the many yeasts, Saccharomyces cerevisiae has been mostly used as a probiotic or prebiotic product in poultry diets (Hooge et al., 2004).
Several yeast products and yeast-constituted feed ingredients are industrially produced, encouraged, and used globally in animal and poultry feed.
![]() Extensive research has been focused on investigating the probable growth performance of the animals and health benefits derived from the addition of yeasts, yeast-containing products, and yeast-based components in feeds.
Extensive research has been focused on investigating the probable growth performance of the animals and health benefits derived from the addition of yeasts, yeast-containing products, and yeast-based components in feeds.
- ⇰ Live dry yeasts are used most frequently, alone or in combination with useful microbes in the form of probiotic products.
- ⇰ Nutritional yeast isolates are used as additives because of their comparatively high protein, amino acid, macro- and micronutrient content in contrast with common feeds and oilseed meals.
- ⇰ Significant yeast-based products include nutraceutical compounds that are present in the yeast cells and cell walls, such as β-glucans, mannooligosaccharides and nucleotides, that have usually been revealed to promote animal growth and health
- (Shruthi et al., 2022; Shurson, 2018)
Yeasts and their components as additives as mycotoxin binders
Toxins produced by fungi are an intrinsic problem in feed production and animal nutrition.
![]() Supplementation of contaminated feed with mycotoxin binders has been considered as the most effective nutritional strategy to decrease the negative impact of mycotoxins.
Supplementation of contaminated feed with mycotoxin binders has been considered as the most effective nutritional strategy to decrease the negative impact of mycotoxins.
Yeasts and their cell wall derivatives also seem to have some capacity to bind mycotoxins like T-2 toxin, aflatoxins, ochratoxin A, and zearalenone, contributing to mitigating their negative effects on animal health and performance (Shetty and Jespersen, 2006).
Some investigations show that mycotoxin mitigation through this strategy is achieved by the adhesion of the mycotoxins to the yeast cell wall components (mannans and β-glucans), not through covalent binding or metabolism, as the dead cells do not lose their binding potentials (Baptista et al., 2004; Celyk et al., 2003).
Some examples of the efficacy of yeast-based products are presented as follows:
A method was developed to evaluate the efficacy of yeast components for the mitigation of three mycotoxins: zearalenone (ZEN), ochratoxin A (OTA), and aflatoxin B1 (AFB1).
⇰ The cell wall of baker’s yeast, was able to adsorb 68% of ZEN, 62% of OTA and 29% of AFB1 (Joannis-Cassan et al., 2011).
In another study, the capacity of yeast cell wall extracts (YCWE) to adsorb ochratoxin (OTA) was assessed. Birds were exposed to five dietary therapies (without OTA, with OTA, and with OTA supplemented with YCWE at three different dosages).
⇰ Deposition of OTA in the liver was evaluated and the data revealed a 30% reduction of the ochratoxin concentrations in the livers of broilers exposed to OTA and supplemented with YCWE (Vartiainen et al., 2020).
The incubation of yeast cell wall with mycotoxins dissolved in a buffer solution caused an unpredicted maximum adsorption when the examination was based only on residual mycotoxins in the supernatant.
⇰ ZEN adsorption was effective at pH 5 (75%), in contrast with pH 3 (60%) and pH 7. OTA was substantially adsorbed at pH 3 (50%) (Faucet-Marquis et al., 2014).
In a study conducted to assess the capacity of heat-killed baker’s yeast (HKBY), cell wall (13)-b-D-glucan of baker’s yeast (BGBY) and cell wall of baker’s yeast (CWBY) to bind AFB1 in phosphate-buffered saline (PBS) spiked with 0.5 μg/mL AFB1.
⇰ Binding of AFB1 to BGBY, CWBY, and HKBY ranged from 6.30 to 46.34%. The maximum binding potential was observed with BGBY with an interaction time of 24 h. Among these binders, CWBY had the maximum binder-AFB1 complex stability after washing with PBS. An outcome of this investigation was that BGBY was the most effective binder as maximum exposure to BGBY eliminated more AFB1 (Aazami et al., 2018).
Crop mannan (CM) has been found to improve bird performance. When birds were exposed to E. coli, CM supplementation in feed was capable of maintaining a growth performance of the birds similar to the growth of birds fed with commercial mannan oligosaccharides or antibiotics.
⇰ The supplementation of mannan oligosaccharides guarded the birds from the wet droppings when confronted with E. coli and aflatoxin contamination (Sundu et al., 2012).
The efficiency of Pichia kudriavzevii as a new yeast bioadsorbent for adsorption AFB1 was assessed, revealing supplementation of P. kudriavzevii (0.1%) in broiler feeds containing AFB1 to be effective (Magnoli et al., 2017).
Furthermore, a study investigated β-D-glucan biopolymers as a mycotoxin binder for DON and FUM, as well as their effect on the nutritional value of soybean, which is considered one of the main raw materials for feed manufacturing.
⇰ The efficiency of β-D-glucan biopolymers on FUM and DON absorption was superior than clay and calcium propionate.
⇰ In vivo treating soyabean seeds with β-D-glucan biopolymers resulted in the reduction in the level of FUM and DON in seeds inoculated artificially with F. verticillioides. Additionally, a low effect on the nutritional components of the β-D-glucan treated seeds when compared to the untreated ones (El-Naggar y Thabit, 2014).
In vitro assessment of the mycotoxin-binding capacity of the live and heat-inactivated Kluyveromyces marxianus QKM-4 cells showed a decrease in OTA and DON concentrations (58% and 49%, respectively) in artificially contaminated buffers.
Yeasts as antifungal agents
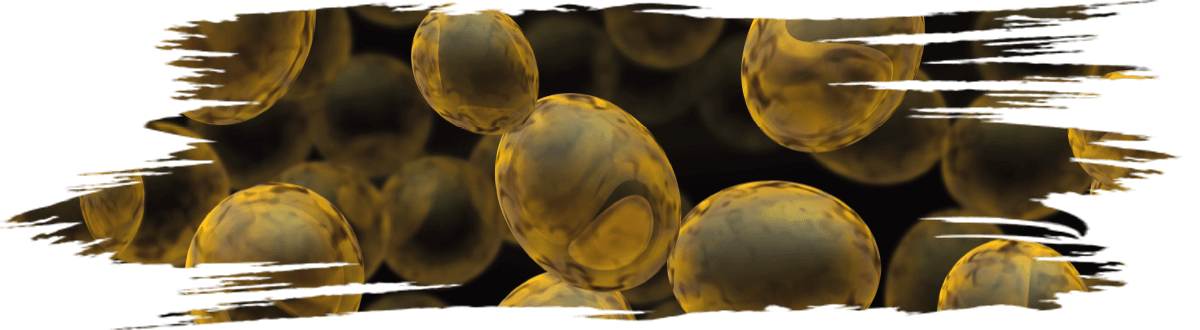
Many yeast strains isolated from various sources have been evaluated to control the growth of fungi (Figure 2).
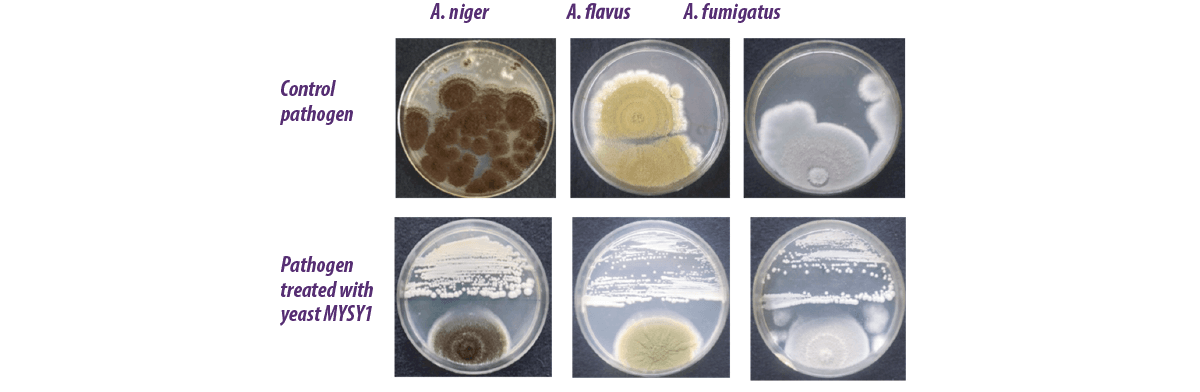
Figure 2. Antifungal activity of yeast MYSY1 against mycotoxin-producing Aspergillus species.
Various mechanisms are involved in the properties of yeasts, such as:
- ⇰ Secretion of enzymes
- ⇰ Competition for nutrients and space
- ⇰ Production of toxins
- ⇰ Release of organic compounds (VOCs)
- ⇰ Induction of resistance in plants
(Delali et al., 2021)
VOCs produced by yeasts have been used to control different fungi associated with feeds (Figure 3).
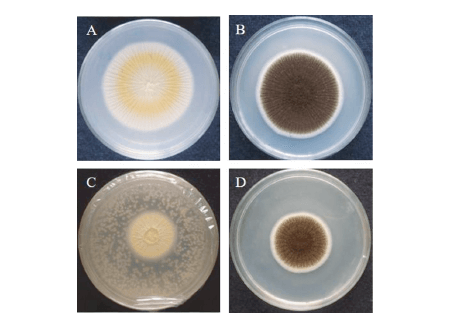
Figure 3. Reduction in the growth of A. niger by the antagonistic activity of VOCs produced by the yeast MYSY1. A and B. Control pathogen plates. C and D. Pathogen plates treated with yeast isolate.
Some examples of the studies involving VOCs are presented below:
To evaluate the production of VOCs by Pichia and its consequence on Monascus purpureus, a double-dish system method was followed.
⇰ The reduction in the growth of M. purpureus was 39.22% after 7 days. As to antifungal volatile compounds, 2-phenylethanol was identified to have a fungicidal effect on M. purpureus through contact and without contact (Zhang et al., 2021).
In another study, a 76.0% and 65.8% reduction were observed in the growth of M. fructicola when exposed to Pichia membranaefaciens and Kluyveromyces apiculata, respectively.
⇰ The VOCs produced by P. membranaefaciens and K. apiculata had a substantial repressive effect on M. fructicola in vitro e in vivo (Zhang et al., 2017).
VOCs from Wickerhamomyces anomalus, Metschnikowia pulcherrima, and Saccharomyces cerevisiae yeast isolates reduced growth of Aspergillus carbonarius, Monilinia fructicola, Penicillium digitatum Alternaria alternata, Botrytis cinerea, Cladosporium spp., and Colletotrichum spp. (Oro et al., 2017).
Wickerhamomyces anomalus, Metschnikowia pulcherrima, Aureobasidium pullulans, and Saccharomyces cerevisiae yeasts were assessed for their antagonistic features to endure and produce antifungal VOCs, both in vivo and in vitro conditions, when inmobilized on commercial hydrogel spheres.
⇰ VOCs produced in vitro by examined yeasts repressed Botrytis cinerea, Penicillium digitatum, and P. italicum mycelial growth and the conidial germination with the maximum hostile activity disclosed for W. anomalus and A. pullulans strains (Prafati et al., 2016).
Three yeasts, Pichia kudriavzevii, Pichia occidentalis, and Meyerozyma guilliermondii/Meyerozyma caribbica, where evaluated against common plant pathogens Mucor spp., Penicillium chrysogenum, Penicillium expansum, Aspergillus flavus, Fusarium cereals, Fusarium poae, and Botrytis cinerea.
Acetate ester, ethyl esters of medium-chain fatty acids and phenylethyl alcohol were among the VOCs produced by the yeasts in the presence of the plant pathogens (Choinska et al., 2020).
Effect of yeasts and their derivatives on poultry
Prebiotic and probiotic properties
![]() A prebiotic is a non-digestible nutrient that beneficially affects the host by selectively stimulating the growth and/ or activity of one or a limited number of bacteria in the gut and, thus, boosting its health (Roto et al., 2015).
A prebiotic is a non-digestible nutrient that beneficially affects the host by selectively stimulating the growth and/ or activity of one or a limited number of bacteria in the gut and, thus, boosting its health (Roto et al., 2015).
The use of antimicrobials as the growth promoters (AGP) in livestock trade has been restricted in several nations, so interest has raised to find substitutes such as prebiotics and other supplements.
In a study conducted to evaluate nutritional strategies using molasses yeast-based prebiotics from Saccharomyces cerevisiae yeast cell wall and autolyzed yeast in substitution of AGP on broiler performance, and phosphorus and nitrogen content in litter after 41 days concluded that yeast- based prebiotics are capable of replacing the AGP without affecting growth performance. Additionally, this supplementation resulted in a higher breast yield (Forniezer et al., 2017).

A significant method to confront these challenges is to use probiotics as an alternative to chemotherapeutics and drugs, acting either individually or in combination with beneficial microbes that influence the host and its performance in numerous ways:
 Repression of fungal pathogens through antagonistic metabolite production, competition for adhesion sites, competition for nutrients, and modification of enzymatic activity of pathogenic bacteria.
Repression of fungal pathogens through antagonistic metabolite production, competition for adhesion sites, competition for nutrients, and modification of enzymatic activity of pathogenic bacteria. Immuno-stimulation and improved natural resistance to communicable diseases in the alimentary tract and prevention of cancer.
Immuno-stimulation and improved natural resistance to communicable diseases in the alimentary tract and prevention of cancer. Nutritional assistance, such as refining food and feed digestibleness through the production of exoenzymes like phytase, amylase, lactase, etc., thus increasing growth rate.
Nutritional assistance, such as refining food and feed digestibleness through the production of exoenzymes like phytase, amylase, lactase, etc., thus increasing growth rate. Increased milk, yield and quality, meat quality, and improved egg production.
Increased milk, yield and quality, meat quality, and improved egg production.
(Vohra et al., 2016)
To achieve these purposes, a probiotic must:
- ⇰ Overcome obstacles in the gut (acidic habitat and high bile contents of the digestive tract).
⇰ Be able to adhere and colonize the digestive tract. - ⇰ Multiply at a maximum rate and have prolonged survivability.
(Merchan et al., 2020)
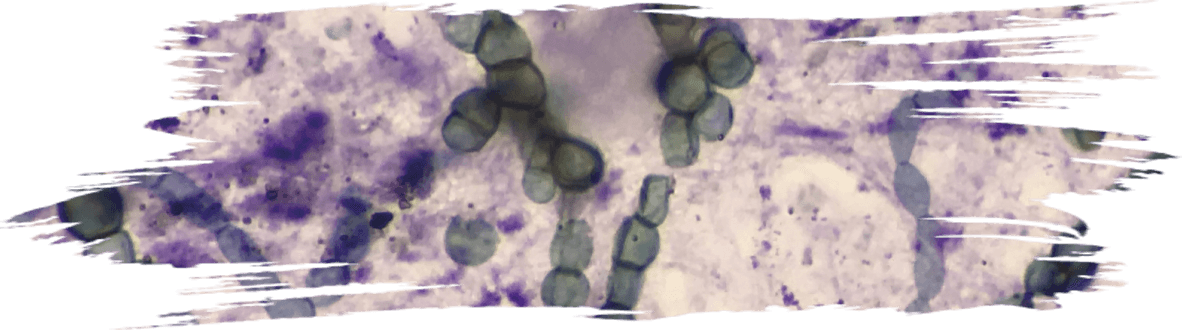
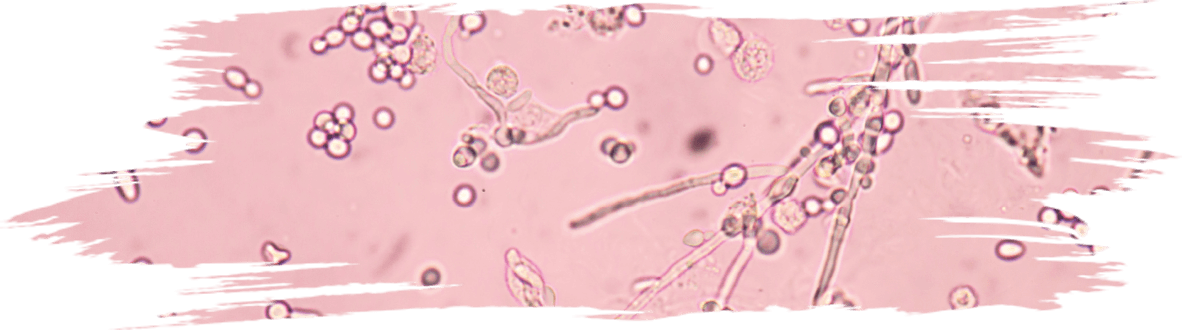
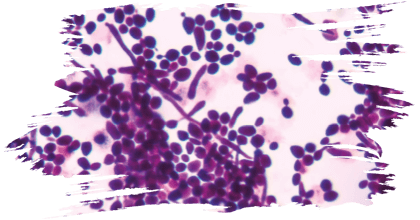
Some of the reported probiotic yeasts isolated from fermented foods and products (Figure 4) are listed in Table 1.
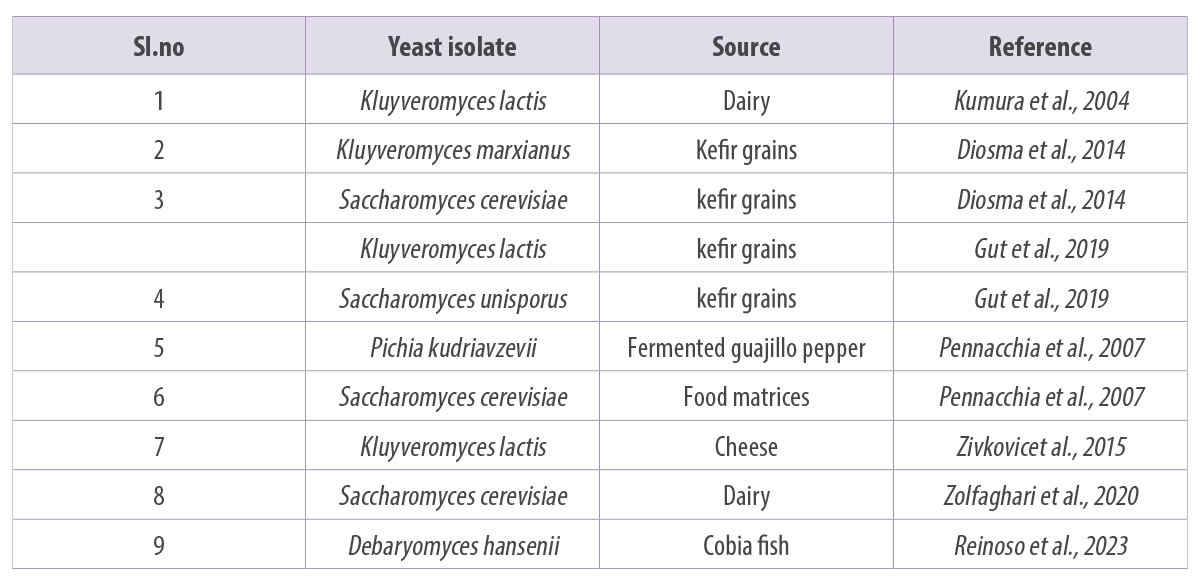
Table 1. Some of the reported yeast probiotics from different sources.
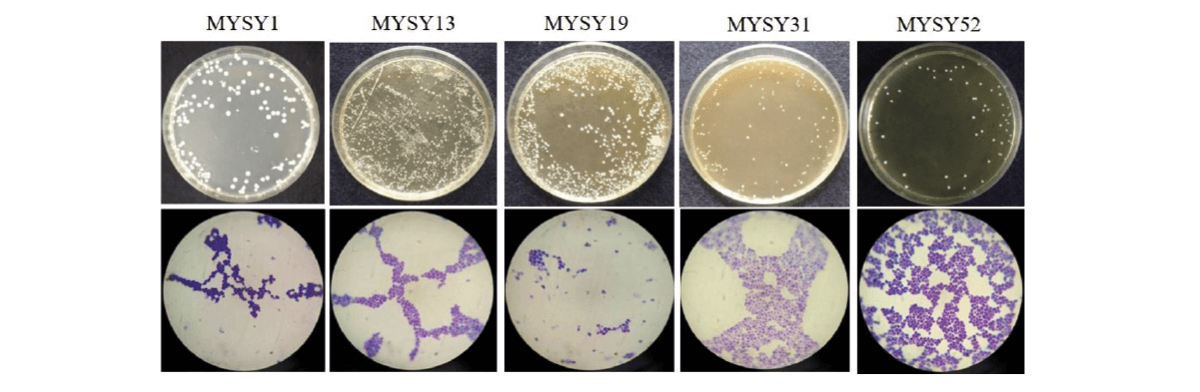
Figure 4. Yeasts isolated from various traditional fermented foods/ products such as Millet’s batter, green
ram batter, Neera, Toddy, Ragi ambli, homemade wines, etc., and their microscopic images.
Alternatives to antibiotics
The need to meet the rising consumer demands calls for the use of preventive antibiotics to attain maximum production.
Antibiotics upgrade food safety and animal well-being by combating or eradicating pathogens. However, due to concerns regarding antibiotic resistance, limitations on the use of antibiotics in food and animal production is obligatory. Henceforth, there is a need to find potential alternatives to antibiotics (Fathima et al., 2023).
Currently, the interest of poultry researchers and producers to identify appropriate alternatives to antibiotics has increased with the prohibition on the use of antibiotics in feed for broilers in some regions of the world and increasing consumer demand for antibiotic-free poultry products.
⇰ One of the proposed alternatives are yeast-derived products (Ahiwe et al., 2021).
The effect of yeast cell wall-derived products, such as mannanoligosaccharides (MOS), has been evaluated in numerous in vivo studies to determine their prebiotic effects on animal performance in the presence of viral or bacterial infections.
Mannoproteins and their branched carbohydrate regions are responsible for pathogen-host recognition and for relation with the environment, and they determine the immunologic specificity of the yeast (Santovito, et al., 2018).
Several studies have demonstrated the outcome of live yeast cells and products derived from YCW in reducing the incidence of infectious diseases in farm animals and improving their innate immune response, therefore vindicating their use as an active replacement approach.
- ⇰ Dietetic supplementation with YCWs has been confirmed to stimulate the systemic innate immunity of broilers (Sadeghi et al., 2013), suggesting the role of YCWs in regulating immune homeostasis (Alizadeh et al., 2016).
- ⇰ The repressive effect of YCW products against infective bacterial adhesion to the mucosal surface of the intestine is apparently due to the capacity of certain bacteria with mannose-binding fimbriae, such as E. coli, Salmonella typhimurium, and Salmonella typhi, to bind to mannoproteins within YCW (Tiago et al., 2012).
Immunomodulation properties
Several studies have reported that the immune response in poultry can be stimulated by the supplementation of yeast derivatives.
The effects of whole Saccharomyces cerevisiae yeast (HY), which are enzymatically hydrolyzed, and yeast cell wall (YCW) pellets on production traits were assessed.
⇰ Humoral immune responses against Newcastle disease virus, histomorphology of the small bowel and microbiology of Ross 308 broiler chickens were also examined, and the results showed that YCW and HY enhanced growth and feed efficacy in broilers. In view of the improvement in the production traits and humoral immune responses, YCW could be better than the HY as a performance enhancer for broilers (Muthuswamy et al., 2011).
A study was conducted to assess the effect of yeast culture (YC) as supplements in broiler diets on performance, digestibility, mucosal development, and immunomodulatory features.
⇰ The yeast amplified antibody levels against Newcastle disease virus, serum lysozyme activity, IgM and IgA levels in the duodenum. Results of this research specify that YC as dietary supplement at 2.5 g/kg enhanced growth performance (Gao et al., 2008).
Another study assessed the consequence of yeast-derived components on serum antibody levels, mRNA gene expression of pattern recognition receptors, growth performance, and cytokines in the broilers.
⇰ Regarding humoral immunity, the YCW diet supplement improved serum IgA levels in comparison to the antibiotic-treated group. Additionally, the YCW diet supplement enhanced the expression of all cytokines and the expression of IFN-γ was upregulated in the DDGS group (supplemented with 8% distiller’s dried grains with solubles (Alizadeh et al., 2016).
Antioxidant properties
Active yeasts are extensively used to improve poultry production and decrease feed costs.
Saccharomyces cerevesiae (SC) significantly reduced total antioxidant capacity (TAC), alanine aminotransferase (ALT), malondialdehyde (MAD), and aspartate aminotransferase (AST) when compared with the standard set, as well as enhancing haemoglobin (Hgb), red blood cells (RBCs), packed cell volume (PCV), phagocytic index (PI), heterophils, monocytes, lymphocytes, and immunity against Avian influenza and Newcastle Disease virus.
⇰ Supplementing with regular or low-density SC diets (0.02% or 0.04%) enhanced the antioxidant features, immunity and the production index of broilers challenged with stressful conditions and contagious agents (Attia et al., 2022).
Another study revealed that the byproducts of β-D-glucan caused the improvement of tumor necrosis factor α (TNF-α) released from murine macrophages and a synergetic effect with cyclophosphamide in the treatment of Lewis lung carcinoma and two types of lymphosarcoma in murine models.
⇰ The outcomes show substantial defensive antigenotoxic antioxidant and antimutagenic activities of the yeast polysaccharides and suggest their probable application in antitumor preclusion/therapy (Kogan et al., 2008).

![]() Future prospects
Future prospects
Until now, maximum mycotoxin adsorption application assessments conducted in the nutrition industry have focused mainly on the application of natural probiotic strains from Saccharomyces cerevisiae and LABs.
Noticeably, high-efficacy and non-polluting mycotoxin-adsorbing strain development strategies have comprehensive research prospects.
Genetic manipulation technology is one of the most promising possibilities to improve mycotoxin adsorption efficiency after the selection of effective adsorption strains, but it requires understanding the molecular mechanisms through which yeasts act against mycotoxins (Walter et al., 2015).
In addition to the genetic manipulation techniques, hybridization methods are viewed as a potentially useful strategy to enhance numerous physiological characteristics as it allows strains to reveal other physiological features that differ from their parent species (Luo et al., 2020).


Conclusions
Currently, yeasts and their components are gaining enormous interest due to their potential as feed additives.
A huge number of investigations and evaluations reported that yeasts and their derivatives may confer advantageous effects on animal growth performance and well-being.
REFERENCES
Aazami, M. H., Nasri, M. H. F., Mojtahedi, M., & Mohammadi, S. R. (2018). In vitro aflatoxin B1 binding by the cell wall and (1→3)-β-D-glucan of Baker’s yeast. Journal of Food Protection, 81(4), 670–676. https://doi.org/10.4315/0362-028X.JFP-17-412
Adithi G, Rakesh Somashekaraiah, S. Divyashree, B. Shruthi, MY Sreenivasa* 2022. Assessment of probiotic and antifungal activity of Lactiplantibacillus plantarum MYSAGT3 isolated from locally available herbal juice against mycotoxigenic Aspergillus species. Food Bioscience. 102118. https://doi.org/10.1016/j.fbio.2022.102118
Ahiwe, E. U., Tedeschi Dos Santos, T. T., Graham, H., & Iji, P. A. (2021). Can probiotic or prebiotic yeast (Saccharomyces cerevisiae) serve as alternatives to in-feed antibiotics for healthy or disease-challenged broiler chickens?: a review. Journal of Applied Poultry Research, 30(3), 100164. https://doi.org/10.1016/j.japr.2021.100164
Attia, Y. A., Al-Khalaifah, H., Abd El-Hamid, H. S., Al-Harthi, M. A., Alyileili, S. R., & El-Shafey, A. A. (2022). Antioxidant Status, Blood Constituents and Immune Response of Broiler Chickens Fed Two Types of Diets with or without Different Concentrations of Active Yeast. Animals, 12(4). https://doi.org/10.3390/ani12040453
Chatterjee, R. N., & Rajkumar, U. (2015). an Overview of Poultry Production in India. Anim. Hlth, 54(2), 89 108
Choińska, R., Piasecka-Jóźwiak, K., Chabłowska, B., Dumka, J., & Łukaszewicz, A. (2020). Biocontrol ability and volatile organic compounds production as a putative mode of action of yeast strains isolated from organic grapes and rye grains. Antonie van Leeuwenhoek, International Journal of General and Molecular Microbiology, 113(8), 1135–1146.
Çelýk, K., Denlý, M., & Savas, T. (2003). Reduction of Toxic Effects of Aflatoxin B 1 by Using Baker Yeast ( Saccharomyces cerevisiae ) in Growing Broiler Chicks Diets Redução dos Efeitos Tóxicos da Aflatoxina B1, Utilizando-se Levedura de Panificação ( Saccharomyces cerevisiae ), na Dieta de. R. Bras. Zootec., 3(1979), 615–619.
Delali ,K.I., Chen ,O., Wang ,W., Yi ,L. , Deng ,L., &Zeng,K.(2021). Evaluation of yeast isolates from kimchi with antagonistic activity against green mold in citrus and elucidating the action mechanisms of three yeast: P. kudriavzevii, K. marxianus, and Y. lipolytica. Postharvest Biology and Technology. 176. https://doi.org/10.1016/j.postharvbio.2021.111495
Deepa ,N., Achar,P.,Sreenivasa,M,Y.,(2021)Current perspectives of biocontrol agents for managementof Fusarium verticilloides and its fumonisin in cereals-A review.Journal of fungi.7,776
Deepa ,N., Charith Raj, A, P., & Sreenivasa, M ,Y., ( 2016). Nested PCR method for the early detection of fumonisin producing Fusarium verticillioides in pure cultures, cereal samples and plant parts. Food Biotechnology. 30(1): 18-29
Deepa ,N,, Rakesh ,S., &Sreenivasa,m,Y,.(2018). Morphological, pathological and mycotoxicological variations among Fusarium verticillioides isolated from cereals. 3 Biotech 8:105 https://doi.org/10.1007/s13205-018 1136-z
Deepa ,N., Achar,P.,Sreenivasa,M,Y.,(2021)Current perspectives of biocontrol agents for management of Fusarium verticilloides and its fumonisin in cereals-A review.Journal of fungi.7,776
Diosma, G., Romanin, D. E., Rey-Burusco, M. F., Londero, A., & Garrote, G. L. (2014). Yeasts from kefir grains: Isolation, identification, and probiotic characterization. World Journal of Microbiology and Biotechnology, 30(1), 43–53. https://doi.org/10.1007/s11274-013-1419-9
El-Naggar, M. A., & Thabit, T. M. (2014). Evaluation of β-d-Glucan biopolymer as a novel mycotoxin binder for fumonisin and deoxynivalenol in soybean feed. Foodborne Pathogens and Disease, 11(6), 433–438. https://doi.org/10.1089/fpd.2013.1711
Fathima, S., Shanmugasundaram, R., Sifri, M., & Selvaraj, R. (2023). Yeasts and yeast-based products in poultry nutrition. Journal of Applied Poultry Research, 32(2), 100345. https://doi.org/10.1016/j.japr.2023.100345
Faucet-Marquis, V., Joannis-Cassan, C., Hadjeba-Medjdoub, K., Ballet, N., & Pfohl-Leszkowicz, A. (2014). Development of an in vitro method for the prediction of mycotoxin binding on yeast-based products: Case of aflatoxin B1, zearalenone and ochratoxin A. Applied Microbiology and Biotechnology, 98(17), 7583–7596. https://doi.org/10.1007/s00253-014-5917-y
Fornazier,R., D. J. Rodrigues , V. Ribeiro J, L. F. T. Albino , F. C. Tavernari , H. S. Rostagno , D. Ladeira , & R. Hernandes(2017)Effects of yeast based prebiotics as replacers to antimicrobial growth promoters in broiles’ diets.ReuniãoAnual da Sociedade Brasileira de Zootecnia
Gao, J., Zhang, H. J., Yu, S. H., Wu, S. G., Yoon, I., Quigley, J., Gao, Y. P., & Qi, G. H. (2008). Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poultry Science, 87(7), 1377–1384. https://doi.org/10.3382/ps.2007-00418
Gut,A.K. , Vasiljevic,T. , Yeager,T. , & Donkor,O.N.,(2019). Characterization of yeasts isolated from traditional kefir grains for potential probiotic properties. Journal of Functional Foods, 58 , 56–66, https://doi.org/10.1016/j.jff.2019.04.046
Hooge, D. M. (2003). Dietary mannan oligosaccharide improves broiler performance. World Poultry, 19(4), 14–15.
Haque, M. A., Wang, Y., Shen, Z., Li, X., Saleemi, M. K., & He, C. (2020). Mycotoxin contamination and control strategy in human, domestic animal and poultry: A review. Microbial Pathogenesis, 142(February), 104095. https://doi.org/10.1016/j.micpath.2020.104095
Kogan, G., Pajtinka, M., Babincova, M., Miadokova, E., Rauko, P., Slamenova, D., & Korolenko, T. A. (2008). Yeast cell wall polysaccharides as antioxidants and antimutagens: Can they fight cancer? Neoplasma, 55(5), 387–393.
Kumura, H., Tanoue, Y., Tsukahara, M., Tanaka, T., & Shimazaki, K. (2004). Screening of dairy yeast strains for probiotic applications. Journal of Dairy Science, 87(12), 4050–4056. https://doi.org/10.3168/jds.S0022-0302(04)73546-8
Lara-Hidalgo, C. E., Dorantes-Álvarez, L., Hernández-Sánchez, H., Santoyo-Tepole, F., Martínez-Torres, A., Villa-Tanaca, L., & Hernández-Rodríguez, C. (2019). Isolation of Yeasts from Guajillo Pepper (Capsicum annuum L.) Fermentation and Study of Some Probiotic Characteristics. Probiotics and Antimicrobial Proteins, 11(3), 7464.
Luo, Y., Liu, X., Yuan, L., & Li, J. (2020). Complicated interactions between bio-adsorbents and mycotoxins during mycotoxin adsorption: Current research and future prospects. Trends in Food Science and Technology, 96(November 2019), 127–134. https://doi.org/10.1016/j.tifs.2019.12.012
Magnoli, A. P., Poloni, V. L., & Cavaglieri, L. (2019). Impact of mycotoxin contamination in the animal feed industry. Current Opinion in Food Science, 29, 99–108. https://doi.org/10.1016/j.cofs.2019.08.009
Merchán, A. V., Benito, M. J., Galván, A. I., & Ruiz-Moyano Seco de Herrera, S. (2020). Identification and selection of yeast with functional properties for future application in soft paste cheese. LWT, 124. https://doi.org/10.1016/j.lwt.2020.109173
Muthusamy, K., Soundharrajan, I., Srisesharam, S., & Kim, D. (n.d.). applied sciences Probiotic Characteristics and Antifungal Activity of Lactobacillus plantarum and Its Impact on Fermentation of Italian Ryegrass at Low Moisture.
Sreenivasa,M,Y., M. T. Gonzalez – Jaen,M,T.,, Regina Sharmila Dass, A.P. Charith Raj, & G. R. Janardhana. (2008). PCR-based assay for the detection and differentiation of potential fumonisin producing F. verticillioides isolated from Indian maize kernels. Food Biotechnology, 22: 160-170.
Sreenivasa, M,Y., Regina Sharmila Dass, Charith Raj ,A,P.,& Janardhana,G,R.,. (2008). PCR-based detection of genus Fusarium and Fumonisin-producing isolates from freshly harvested Sorghum grains grown in
Karnataka, India. Journal of Food Safety, 28: 236- 237
Nagaraja , H., Chennappa, G., Rakesh, S., Naik, M,K., Amaresh Y,S., and Sreenivasa, M. Y. , (2016). Antifusarial activity of Azotobacter nigricans against trichothecene-producing Fusarium species associated with cereals. Food Science and Biotechnology. 25(4): 1197- 1204. DOI 10.1007/s10068-016-0.
Oro, L., Feliziani, E., Ciani, M., Romanazzi, G., & Comitini, F. (2018). Volatile organic compounds from Wickerhamomyces anomalus, Metschnikowia pulcherrima and Saccharomyces cerevisiae inhibit growth of decay causing fungi and control postharvest diseases of strawberries. International Journal of Food Microbiology, 265, 18–22.
Parafati,L., Vitale,A., Restuccia,C., & Cirvilleri,G., (2016) Performance evaluation of volatile organic compounds by antagonistic yeasts immobilized on hydrogel spheres against gray, green and blue postharvest decays. Food Microbiology, doi: 10.1016/j.fm.2016.11.021
Pennacchia, C., Blaiotta, G., Pepe, O., & Villani, F. (2008). Isolation of Saccharomyces cerevisiae strains from different food matrices and their preliminary selection for a potential use as probiotics. Journal of Applied Microbiology, 105(6), 1919–1928. https://doi.org/10.1111/j.1365-2672.2008.03968.x
Pfliegler, W. P., Pusztahelyi, T., & Pócsi, I. (2015). Mycotoxins – prevention and decontamination by yeasts. In Journal of Basic Microbiology (Vol. 55, Issue 7, pp. 805–818). Wiley-VCH Verlag. https://doi.org/10.1002/jobm.201400833
Ravindran.(2013). Poultry development review. Poultry genetics and breeding in developing countries
Reinoso, S.; Gutiérrez, M.S.; Domínguez-Borbor, C.; Argüello-Guevara, W.; Bohórquez-Cruz, M.; Sonnenholzner, S.; Nova-Baza, D.; Mardones, C.; Navarrete, P. (2023) Selection of Autochthonous Yeasts Isolated from the Intestinal Tracts of Cobia Fish (Rachycentron canadum) with Probiotic Potential. Journal of Fungi , 9, 274. https://doi.org/10.3390/jof9020274
Roto, S. M., Rubinelli, P. M., & Ricke, S. C. (2015). An introduction to the avian gut microbiota and the effects of yeast-based prebiotic-type compounds as potential feed additives. Frontiers in Veterinary Science, 2(SEP). https://doi.org/10.3389/fvets.2015.00028
Sadeghi, A. A., Mohammadi, A., Shawrang, P., & Aminafshar, M. (2013). Immune responses to dietary inclusion of prebiotic-based mannan-oligosaccharide and β-glucan in broiler chicks challenged with Salmonella enteritidis. Turkish Journal of Veterinary and Animal Sciences, 37(2), 206–213. https://doi.org/10.3906/vet-1203-9
Sampaio Baptista, A., Horii, J., Antonia Calori-Domingues, M., Micotti Da Glória, E., Mastrodi Salgado, J., & Roberto Vizioli, M. (2004). The capacity of manno-oligosaccharides,
Santovito, E., Greco, D., Logrieco, A. F., & Avantaggiato, G. (2018). Eubiotics for food security at farm level: Yeast cell wall products and their antimicrobial potential against pathogenic bacteria. Foodborne Patho
Sundu ,B., Hatta,U.& Chaudhry , A.S.,(2012). Potential use of beta mannan from copra mannan as afeed additives for broilers. World poultry science journal,68.
Sampaio Baptista, A., Horii, J., Antonia Calori-Domingues, M., Micotti Da Glória, E., Mastrodi Salgado, J., & Roberto Vizioli, M. (2004). The capacity of manno-oligosaccharides,
Shruthi, B. , N. Deepa , Rakesh Somashekaraiah , G. Adithi , S. Divyashree , M Y Sreenivasa. (2022) Exploring biotechnological and functional characteristics of probiotic yeasts: A review. Biotechnology Reports, 34. https://doi.org/10.1016/j.btre.2022.e00716
Shurson, G. C. (2018). Yeast and yeast derivatives in feed additives and ingredients: Sources, characteristics, animal responses, and quantification methods. Animal Feed Science and Technology, 235(November 2017), 60–76. https://doi.org/10.1016/j.anifeedsci.2017.11.010
Tiago, F. C. P., Martins, F. S., Souza, E. L. S., Pimenta, P. F. P., Araujo, H. R. C., Castro, I. M., Brandão, R. L., & Nicoli, J. R. (2012). Adhesion to the yeast cell surface as a mechanism for trapping pathogenic bacteria by Saccharomyces probiotics. Journal of Medical Microbiology, 61(PART 9), 1194–1207. https://doi.org/10.1099/jmm.0.042283-0
Vartiainen, S., Yiannikouris, A., Apajalahti, J., & Moran, C, A. (2020) Comprehensive Evaluation of the Efficiency of Yeast Cell Wall Extract to Adsorb Ochratoxin A and Mitigate Accumulation of the Toxin in Broiler Chickens. Toxins ,12(37), doi:10.3390/toxins12010037
Vohra, A., Syal, P., & Madan, A. (2016). Probiotic yeasts in livestock sector. Animal Feed Science and Technology, 219, 31–47. https://doi.org/10.1016/j.anifeedsci.2016.05.019
Walker et al., 1995)Walker, G. M., Mcleod, A. H., & Hodgson, V. J. (1995). Interactions between killer yeasts and pathogenic fungi. FEMS Microbiology Letters, 127(3), 213–222. https://doi.org/10.1016/0378 1097(95)00064-C
Zhang, H., Du, H., & Xu, Y. (2021). Volatile Organic Compound-Mediated Antifungal Activity of Pichia spp. and Its Effect on the Metabolic Profiles of Fermentation Communities. Applied and Environmental Microbiology, 87(9), 1–15. https://doi.org/10.1128/AEM.02992-20
Zhang, J., Xie, J., Zhou, Y., Deng, L., Yao, S., & Zeng, K. (2017). Inhibitory effect of Pichia membranaefaciens and Kloeckera apiculata against Monilinia fructicola and their biocontrol ability of brown rot in postharvest plum. Biological Control, 114, 51–58. https://doi.org/10.1016/j.biocontrol.2017.07.013
Zivkovic, M., Cadez, N., Uroic, K., Miljkovic, M., Tolinacki, M., Dousova, P., Kos, B., Suskovic, J., Raspor, P., Topisirovic, L., & Golic, N. (2015). Evaluation of probiotic potential of yeasts isolated from traditional cheeses manufactured in Serbia and Croatia. Journal of Intercultural Ethnopharmacology, 4(1), 12. https://doi.org/10.5455/jice.20141128051842
Zolfaghari,H. , Khezerlou,A., Ehsani,A., & Khosroushahi.A.Y.,(2020). Detoxification of Aflatoxin B1 by Probiotic Yeasts and Bacteria Isolated From Dairy Products of Iran Advance Pharmaceutical Bulletin,, 10(3), 482-487 ,doi: 10.34172/apb.2020.060




 Micotoxicosis prevention
Micotoxicosis prevention