Luis-Miguel Gómez-Osorio. DVM, MSc, PhD
Regional Technical Manager PATENT CO & agromed CIBAV Research Group, Facultad de Ciencias Agrarias, Universidad de Antioquia, Colombia
The poultry industry in South America faces a significant challenge from mycotoxins, which are harmful secondary metabolites produced by molds, that compromise poultry health, productivity, and economic stability.
The region’s diverse climatic conditions, exacerbated by climate change, promote the prevalence of key mycotoxins such as aflatoxins, fumonisins, ochratoxins, zearalenone, trichothecenes, among others, which are detrimental to poultry and public health.
The lack of harmonized standards, inadequate laboratory infrastructure, and limited farmer awareness compound these issues, particularly affecting smallholders who bear disproportionate economic burdens.
Strategies such as the use of advanced mycotoxin adsorbents, including clinoptilolite-based products and biocontrol agents such as Bacillus species, show promise in reducing mycotoxin toxicity and safeguarding poultry health.

 THE THREAT OF MYCOTOXINS TO THE SOUTH AMERICAN POULTRY SECTOR
THE THREAT OF MYCOTOXINS TO THE SOUTH AMERICAN POULTRY SECTOR
South America (SA) is the home to some of the world’s largest poultry producers, faces a persistent challenge: managing the risks posed by mycotoxins1.
This region spans an impressive area of 17,840,000 square kilometers (6,890,000 square miles).
Reflecting its vast size, the continent stretches from a wide equatorial region in the north to a slender sub-Arctic zone in the south.
The climate across SA is notably diverse, ranging from tropical and temperate to arid and frigid conditions2.

Mycotoxins lead to substantial economic losses due to their effects on:
- ⇰ Human and animal health
- ⇰ Animal well-being
- ⇰ Productivity
- ⇰ Local and global trade3
They occur in favorable environments in most raw materials worldwide.
 More than 700 types of mycotoxins have been identified to date mycotoxins have been identified to date, the most important being aflatoxin B1 (AFB1), fumonisin B1 (FB1), deoxynivalenol (DON), ochratoxin A (OTA), zearalenone (ZEN) as well as T-2 toxin, because of their impact on poultry productivity and health4 (Figure 1)5.
More than 700 types of mycotoxins have been identified to date mycotoxins have been identified to date, the most important being aflatoxin B1 (AFB1), fumonisin B1 (FB1), deoxynivalenol (DON), ochratoxin A (OTA), zearalenone (ZEN) as well as T-2 toxin, because of their impact on poultry productivity and health4 (Figure 1)5.
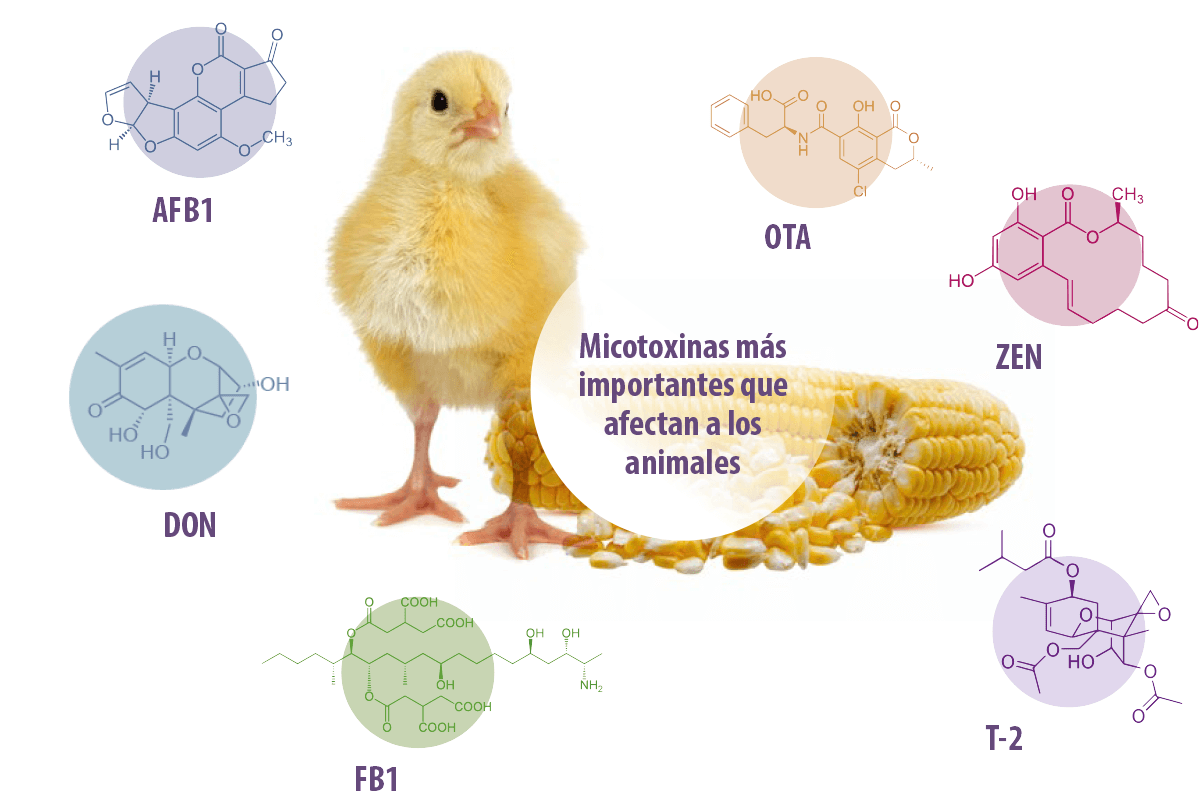
Figure 1. Most important mycotoxins affecting animals. Abbreviations: AFB1: aflatoxin B1, FB1: fumonisin B1, DON: deoxynivalenol, OTA: ochratoxin A, ZEN: zearalenone, T-2: T-2 toxin . (Adapted from Gómez-Osorio and Vasiljević, 2023)6.
These toxic compounds are chemically very stable, resistant to temperature, storage, and processing conditions.
⇰ They originate from filamentous fungi called molds, such as Fusarium sp, Aspergillus sp, Penicillium sp, and they are not essential for their growth7.
Despite the critical nature of this issue, regulatory frameworks for controlling mycotoxin levels in poultry feed are inconsistent across the region.
![]() This lack of uniformity in safety standards creates uncertainty for producers and hinders the development of robust mitigation strategies.
This lack of uniformity in safety standards creates uncertainty for producers and hinders the development of robust mitigation strategies.
CONTAMINATION OF MYCOTOXINS IN THE REGION OF SA
Table 1 shows the prevalence of different mycotoxins in South America.
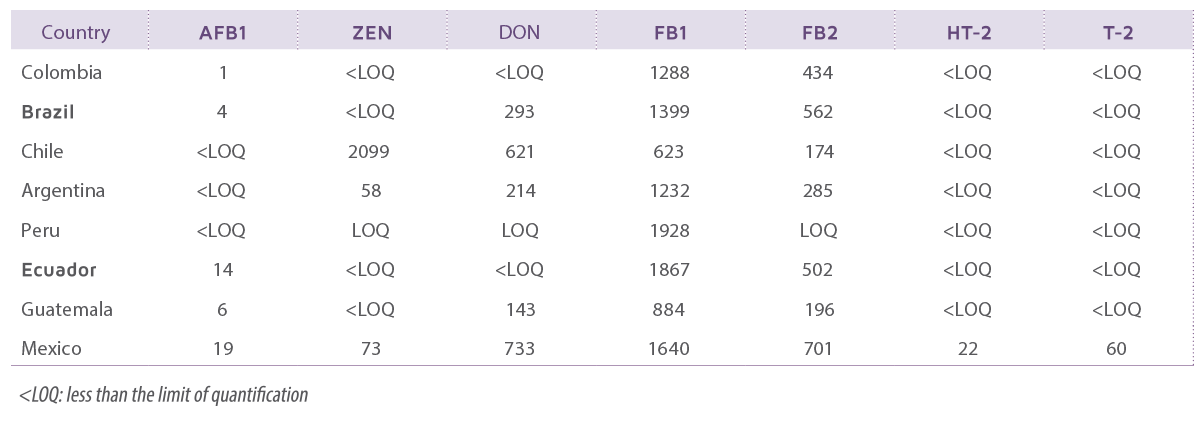
Table 1. Mean of multi-mycotoxin contamination levels detected (ppb) in maize samples in countries of South America during 2020- 2022 (Adapted from Raj et al, 2022a and 2022b8,9).
Mycotoxin contamination poses a significant challenge in SA, affecting staple crops such as corn, wheat, oats, and rye.
The region’s diverse climates, ranging from tropical to temperate, create favorable conditions for the growth of mycotoxin-producing fungi like Aspergillus, Fusarium and Penicillium species.
Una revisión de estudios de 2018 a 2023 destaca la prevalencia de micotoxinas tradicionales, incluidas aflatoxinas, OTA, fumonisinas, DON y ZEN, con ocurrencias que varían según la región y las condiciones climáticas10.
Emerging and masked mycotoxins have been studied primarily in Argentina and Brazil, where some studies have shown high occurrences11,12.
⇰ This underscores the need for enhanced food safety measures and surveillance systems to mitigate mycotoxin risks in SA LATAM countries.
PROBLEMS ASSOCIATED WITH VIGILANCE AND CONTROL OF MYCOTOXINS
The vigilance and control of mycotoxins in SA face numerous challenges, reflecting the region’s economic, climatic, and infrastructural diversity.
Regulations 
One significant issue is the lack of standardized regulations across countries.
While some nations like Brazil and Argentina have developed frameworks for monitoring mycotoxins, other countries such as Colombia lag behind, creating gaps in regional consistency.
⇰ This disparity complicates efforts to establish effective cross-border trade agreements and maintain food safety.
Sampling
Sampling plays a critical role in the vigilance and control of mycotoxins, ensuring the safety and quality of food and feed across supply chains13,14.
Effective sampling methods are the foundation for:
 Detecting contamination levels
Detecting contamination levels Assessing compliance with safety standards
Assessing compliance with safety standards Preventing the circulation of contaminated products in both domestic and international markets15
Preventing the circulation of contaminated products in both domestic and international markets15
Given the uneven distribution of mycotoxins within batches of agricultural products, reliable sampling plans are essential to accurately reflect contamination levels14.
![]() Without robust sampling strategies, even the most advanced analytical methods can yield misleading results, undermining the entire risk management process16.
Without robust sampling strategies, even the most advanced analytical methods can yield misleading results, undermining the entire risk management process16.

Laboratory infrastructure
Laboratory infrastructure is another critical barrier.
Advanced equipment necessary for mycotoxin testing, such as HPLC or ELISA kits, is costly and often unavailable in rural or underfunded areas16,17.
As a result, farmers and small producers struggle to assess contamination levels in their crops, leading to undetected health risks and economic losses.
 Limited access to accredited labs further delays testing and reduces the capacity for timely interventions.
Limited access to accredited labs further delays testing and reduces the capacity for timely interventions.

Education and awareness 
Education and awareness are also insufficient18. Many farmers, especially smallholders, lack knowledge about:
- ⇰ Proper crop storage
- ⇰ Drying techniques
- ⇰ Other preventative measures to overcome fungal growth
Traditional practices, combined with humid climates and poor post-harvest handling, exacerbate the risk of contamination19. Additionally, inadequate government initiatives to disseminate information on mycotoxin management leave vulnerable populations at greater risk.
Enforcement of existing regulations presents its own challenges.
Even in countries with established standards, limited funding and staffing prevent regulatory agencies from conducting thorough inspections or implementing penalties for non-compliance. Corruption and bureaucratic inefficiencies can further weaken these efforts.

Climate change
Climate change is increasingly influencing mycotoxin occurrences in SA by altering the environmental conditions that promote fungal growth20.
Aflatoxin contamination, for instance, becomes more prevalent during droughts, as high temperatures and low rainfall create stress conditions for crops, making them more vulnerable to infection by aflatoxin-producing fungi21.
Conversely, warm and wet climates foster the growth of Fusarium species, which produce deoxynivalenol (DON) and other harmful toxins.
⇰ These fungi thrive in high humidity, particularly in crops such as wheat during flowering and maize during silking22.
In SA, where agricultural regions already contend with varied climatic conditions, the intensification of extreme weather events due to climate change, such as prolonged droughts or excessive rainfall, heightens the risk of mycotoxin outbreaks.
This dual threat not only impacts crop yields but also compromises food and feed safety, posing significant challenges for farmers and food supply chains in the region.
Finally, the economic burden of mycotoxin control disproportionately affects small producers who lack resources to invest in modern farming practices, better storage facilities, or regular testing.
This inequality perpetuates cycles of poverty and food insecurity in rural areas, while wealthier exporters face stricter scrutiny in international markets.
EFFECTS OF MYCOTOXINS ON POULTRY HEALTH AND PERFORMANCE
Mycotoxins significantly affect poultry health and productivity, primarily through their impact on the gastrointestinal tract, immune system, and overall metabolism23.
Aflatoxins, for instance, are known to, inhibit protein synthesis, leading to:
- ⇰ Decreased weight gain
- ⇰ Poor feed conversion rates
- ⇰ Reduced egg production24
 These effects often manifest through liver damage, evident as pale, enlarged livers during necropsy.
These effects often manifest through liver damage, evident as pale, enlarged livers during necropsy.
Chronic exposure to mycotoxins like aflatoxins and ochratoxins also weakens the birds’ immune systems, resulting in increased susceptibility to infections and higher mortality rates25.
Subclinical doses of mycotoxins, while not immediately evident, can still compromise gut health by disrupting nutrient absorption and damaging the intestinal barrier, further impairing the birds’ ability to thrive26.
The immune system is particularly vulnerable to mycotoxin exposure, as toxins like aflatoxins and trichothecenes suppress lymphocyte proliferation and antibody production.
⇰ This leads to poor vaccine responses and higher incidences of diseases such as Newcastle and infectious bursal disease27.
Additionally, toxins such as fumonisins and DON contribute to mucosal damage, increasing intestinal permeability and predisposing birds to gastrointestinal infections, including coccidiosis as well as necrotic enteritis23,28.
 These combined impacts not only reduce flock productivity but also impose significant economic losses on poultry producers, emphasizing the critical need for vigilant monitoring and management of mycotoxin contamination in poultry feed.
These combined impacts not only reduce flock productivity but also impose significant economic losses on poultry producers, emphasizing the critical need for vigilant monitoring and management of mycotoxin contamination in poultry feed.
STRATEGIES FOR EFFECTIVE MYCOTOXIN RISK MANAGEMENT IN THE POULTRY INDUSTRY IN SA
Effective mycotoxin risk management in the poultry industry in SA requires a strategic combination of preventative and mitigative measures based on the most frequent mycotoxins.
Studies such as those on the novel multicomponent mycotoxin detoxifying agent (MYCORAID) and inorganic adsorbents highlight their utility in reducing the adverse effects of mycotoxins like aflatoxins, fumonisins, and ochratoxins25,29.
MYCORAID, which incorporates specially selected minerals, probiotics (e.g., Bacillus strains), plant extracts, and yeast cell walls, has been shown to:
 Improve broiler and layer feed conversion ratios
Improve broiler and layer feed conversion ratios Enhance stabilize gut
Enhance stabilize gut Reduce toxin residue in tissues
Reduce toxin residue in tissues

Similarly, inorganic adsorbents based on clinoptilolite, demonstrated efficacy in improving growth parameters and reducing histopathological damage in poultry exposed to mycotoxin-contaminated diets.
 Preventative strategies include rigorous feed quality controls, such as testing and monitoring for contamination at production and storage stages.
Preventative strategies include rigorous feed quality controls, such as testing and monitoring for contamination at production and storage stages.Climate conditions in South America exacerbate mycotoxin risks, necessitating the use of adsorbents like clinoptilolite and other detoxifying agents to bind toxins and prevent absorption in the gastrointestinal tract.
Research has shown that these interventions:
 Mitigate the immediate toxic effects
Mitigate the immediate toxic effects Help in maintaining optimal immune function and minimizing economic losses associated with reduced poultry productivity30
Help in maintaining optimal immune function and minimizing economic losses associated with reduced poultry productivity30
To achieve sustainable mycotoxin management, an integrated approach is recommended. This includes adopting good agricultural practices (GAP) to minimize fungal contamination pre-harvest, employing biological controls like Bacillus subtilis for toxin bioremediation, and combining them with chemical adsorbents for immediate remediation.
Leveraging local research, such as findings from trials conducted on adsorbents, provides practical insights into the specific challenges of South America’s poultry industry.
 Collaboration among feed producers, farmers, and regulators is key to refining these strategies and ensuring safe, toxin-free poultry production.
Collaboration among feed producers, farmers, and regulators is key to refining these strategies and ensuring safe, toxin-free poultry production.

MYCORAID: AN ADVANCED SOLUTION FOR MYCOTOXIN RISK MANAGEMENT IN POULTRY
MYCORAID represents a breakthrough in mycotoxin management, offering a multilayered defense against a broad spectrum of bio-toxins, including mycotoxins, endotoxins, and algal toxins.
Its innovative formula integrates specially selected minerals, microbial strains, herbal extracts, and yeast cell walls, providing comprehensive protection for poultry wellbeing and productivity.
 The product’s primary mechanisms of action include adsorption, biotransformation, hepatoprotection, and immunomodulation, tailored to meet the unique challenges of mycotoxin contamination in SA.
The product’s primary mechanisms of action include adsorption, biotransformation, hepatoprotection, and immunomodulation, tailored to meet the unique challenges of mycotoxin contamination in SA.
The adsorption layer features a mineral mixture with exceptional selectivity and stability, adsorbing both polar and less-polar mycotoxins such as AFB1, T-2 toxin, as well as FB1 with high efficiency in the gastrointestinal tract.
Complementing this, internationally deposited strains of Bacillus subtilis and Bacillus licheniformis drive biotransformation, converting toxic compounds like OTA and ZEN into less harmful metabolites.
 This process not only neutralizes toxins but also contributes to gut health by promoting a balanced microbiota.
This process not only neutralizes toxins but also contributes to gut health by promoting a balanced microbiota.
Herbal extracts, such as those containing flavolignans like silymarin, play a crucial role in hepatoprotection by:
 Scavenging free radicals
Scavenging free radicals Enhancing liver detoxification
Enhancing liver detoxification Restoring antioxidative enzyme function
Restoring antioxidative enzyme function
Additionally, the yeast cell wall components, rich in β-glucans and mannan oligosaccharides, booster the immune system by stimulating T-cells, B-cells, and macrophages, ensuring robust host disease resistance.
 In vivo trials have demonstrated MYCORAID’s efficacy in improving feed conversion ratios (FCR), reducing toxin residues in tissues, and sustaining poultry performance even under high contamination scenarios29,31,32.
In vivo trials have demonstrated MYCORAID’s efficacy in improving feed conversion ratios (FCR), reducing toxin residues in tissues, and sustaining poultry performance even under high contamination scenarios29,31,32.
 MYCORAID’s unique combination of adsorption, biotransformation, and immunoprotection makes it an indispensable tool for broiler and egg producers across SA, delivering both safety and economic benefits in countries with high occurrence of mycotoxins.
MYCORAID’s unique combination of adsorption, biotransformation, and immunoprotection makes it an indispensable tool for broiler and egg producers across SA, delivering both safety and economic benefits in countries with high occurrence of mycotoxins.
CONCLUSIONS
There is a pressing need for harmonized mycotoxin regulations across South American countries to address inconsistencies that compromise food safety, trade efficiency, and the implementation of effective mitigation strategies in the poultry industry.
SA’s diverse climatic conditions, further intensified by climate change, significantly drive the prevalence of mycotoxins, necessitating tailored, region-specific strategies for monitoring and control.
A key challenge is the lack of adequate laboratory infrastructure and limited access to advanced testing methods, particularly in rural areas, which emphasizes the urgent need for investment in diagnostic and monitoring capabilities.
Compounding the issue, smallholder farmers often lack awareness of preventative measures, such as proper crop storage and drying techniques, which exacerbates the risk of mycotoxin contamination.
⇰ This gap highlights the importance of education and targeted awareness campaigns to empower farmers with the knowledge needed to mitigate contamination risks effectively.
Moreover, small producers bear a disproportionate economic burden in managing mycotoxin risks due to limited resources, underscoring the need for inclusive policies and financial support mechanisms to ensure equitable access to mitigation tools and practices.
To address these challenges, the adoption of advanced mycotoxin adsorbents, including clinoptilolite-based products, and the integration of bioremediation agents such as Bacillus species, offer promising solutions.
These strategies have shown significant potential in reducing the toxic effects of mycotoxins, safeguarding poultry health, and enhancing productivity, making them vital components of a comprehensive approach to mycotoxin risk management.
REFERENCES
1. Chadd S. “Poultry in the 21st Century: Avian Influenza and Beyond.,” Poultry in the 21st Century: Avian Influenza and Beyond. (2007). p. 269–297 http://www.fao.org/docrep/011/i0323e/i0323e00.htm
2. Garreaud RD, Vuille M, Compagnucci R, Marengo J. Present-day South American climate. Palaeogeogr Palaeoclimatol Palaeoecol (2009) 281:180–195. doi: 10.1016/j.palaeo.2007.10.032
3. Pitt JI, David Miller J. A Concise History of Mycotoxin Research. J Agric Food Chem (2017) 65:7021–7033. doi: 10.1021/acs. jafc.6b04494
4. Sulyok M, Suman M, Krska R. Quantification of 700 mycotoxins and other secondary metabolites of fungi and plants in grain products. npj Sci Food (2024) 8:1–8. doi: 10.1038/s41538-024-00294-7
5. Bennett J, Klich M. Mycotoxins. Clin Microbiol Rev (2003) 16:497–516. doi: 10.1016/B978-0-12-415759 0.00039-X
6. Gomez-Osorio L-M, Vasiljević M. Micotoxinas en alimentos. Realidad y prevención en la avicultura moderna. Plumazos (2023) 14:38–45. http://medcontent.metapress.com/index/A65RM03P4874243N.pdf%5Cnhttp://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9135719%5Cnhttps://books.google.com/books?id=hIIzAQAAMAAJ&pgis=1%5Cnhttp://dx.doi.org/10.1016/j.ijfo
7. Cheng Y-L, Lee C-Y, Huang Y-L, Buckner CA, Lafrenie RM, Dénommée JA, Caswell JM, Want DA, Gan GG, Leong YC, et al. “Mycotoxins in Poultry.,” In: Milad Manafi, editor. Poultry Science. Intechopen (2016). p. 13 https://www.intechopen.com/books/advanced-biometric-technologies/liveness-detection-in-biometrics
8. Raj J, Farkaš H, Jakovčević Z, Medina A, Magan N, Čepela R, Vasiljević M. Comparison of multiple mycotoxins in harvested maize samples in three years (2018–2020) in four continents. Food Addit Contam (2022) 39:599 608. doi:10.1080/19440049.2021.2012600
9. Raj J, Farkas H, Cujic S, Jakovcevic Z, Vasiljević M. Higher prevalence of Fumonisins and Fusaric acid in 2022 harvested corn from Asia. (2022) https://mycotoxinsite.com/higher-prevalence-aflatoxins-fumonisins-2021 harvested-corn-asia/?lang=en [Accessed November 26, 2024]
10. Foerster C, Müller-Sepúlveda A, Copetti MV, Arrúa AA, Monsalve L, Ramirez ML, Torres AM. A mini review of mycotoxin’s occurrence in food in South America in the last 5 years: research gaps and challenges in a climate change era. Front Chem Biol (2024) 3:1–8. doi: 10.3389/fchbi.2024.1400481
11. Fraeyman S, Croubels S, Devreese M, Antonissen G. Emerging fusarium and alternaria mycotoxins: Occurrence, toxicity and toxicokinetics. Toxins (Basel) (2017) 9:1–26. doi: 10.3390/toxins9070228
12. Kovalsky P, Kos G, Nährer K, Schwab C, Jenkins T, Schatzmayr G, Sulyok M, Krska R. Co-occurrence of regulated, masked and emerging mycotoxins and secondary metabolites in finished feed and maize–An extensive survey. Toxins (Basel) (2016) 8:1–29. doi: 10.3390/toxins8120363
13. Resnik S, Costarrica ML, Pacin A. Mycotoxins in Latin America and the Caribbean. Food Control (1995) 6:19 28. doi:10.1016/0956-7135(95)91450-Y
14. Coker RD, Nagler MJ, Blunden G, Sharkey AJ, Defize PR, Derksen GB, Whitaker TB. Design of sampling plans for mycotoxins in foods and feeds. Nat Toxins (1995) 3:257–262. doi: 10.1002/nt.2620030417
15. Köppen R, Koch M, Siegel D, Merkel S, Maul R, Nehls I. Determination of mycotoxins in foods: Current state of analytical methods and limitations. Appl Microbiol Biotechnol (2010) 86:1595–1612. doi: 10.1007/s00253-010 2535-1
16. Janik E, Niemcewicz M, Podogrocki M, Ceremuga M, Gorniak L, Stela M, Bijak M. The existing methods and novel approaches in mycotoxins’ detection. Molecules (2021) 26: doi: 10.3390/molecules26133981
17. Agriopoulou S, Stamatelopoulou E, Varzakas T. Advances in analysis and detection of major mycotoxins in foods. Foods (2020) 9:1–23. doi: 10.3390/foods9040518
18. Adekoya I, Njobeh P, Obadina A, Chilaka C, Okoth S, De Boevre M, De Saeger S. Awareness and prevalence of mycotoxin contamination in selected nigerian fermented foods. Toxins (Basel) (2017) 9:1–16. doi:10.3390/toxins9110363
19. Leslie JF, Morris JB, Gurung JK, Harvey JJW, Ayalew A, Baker R, Zhang G. Mycotoxin communications: Managing messages for different audiences. Front Sustain Food Syst (2023) 6: doi: 10.3389/fsufs.2022.1095256
20. Perrone G, Ferrara M, Medina A, Pascale M, Magan N. Toxigenic Fungi and Mycotoxins in a Climate Change Scenario: Ecology , Genomics , Distribution , Prediction and Prevention of the Risk. (2020)1–20.
21. Medina Á, González-Jartín JM, Sainz MJ. Impact of global warming on mycotoxins. Curr Opin Food Sci (2017) 18:76–81. doi: 10.1016/j.cofs.2017.11.009
22. Zingales V, Taroncher M, Martino PA, Ruiz MJ, Caloni F. Climate Change and Effects on Molds and Mycotoxins. Toxins (Basel) (2022) 14:1–13. doi: 10.3390/toxins14070445
23. Gómez-Osorio LM, Vasiljevic M, Raj J, Chaparro-Gutierréz JJ, López-Osorio S. Mycotoxins and coccidiosis in poultry –co-occurrence, interaction, and effects. Front Vet Sci (2024) 11: doi: 10.3389/fvets.2024.1387856
24. Murugesan GR, Ledoux DR, Naehrer K, Berthiller F, Applegate TJ, Grenier B, Phillips TD, Schatzmayr G. Prevalence and effects of mycotoxins on poultry health and performance, and recent development in mycotoxin counteracting strategies. Poult Sci (2015) 94:1298–1315. doi: 10.3382/ps/pev075
25. Vasiljević M, Marinković D, Milićević D, Pleadin J, Stefanović S, Trialović S, Raj J, Petrujkić B, Trialović JN. Efficacy of a modified clinoptilolite based adsorbent in reducing detrimental effects of ochratoxin a in laying hens. Toxins (Basel) (2021) 13: doi: 10.3390/toxins13070469
26. Yang C, Song G, Lim W. Effects of mycotoxin-contaminated feed on farm animals. J Hazard Mater (2020) 389:122087. doi: 10.1016/j.jhazmat.2020.122087
27. Vörösházi J, Neogrády Z, Mátis G, Mackei M. Pathological consequences, metabolism and toxic effects of trichothecene T-2 toxin in poultry. Poult Sci (2024) 103:1–13. doi: 10.1016/j.psj.2024.103471
28. Antonissen G, Van Immerseel F, Pasmans F, Ducatelle R, Haesebrouck F, Timbermont L, Vertinden M, Janssens GPJ, Eeckhaut V, Eeckhout M, et al. The mycotoxin deoxynivalenol predisposes for the development of Clostridium perfringens-induced necrotic enteritis in broiler chickens. PLoS One (2014) 9:1–8. doi:10.1371/journal.pone.0108775
29. Raj J, Farkaš H, Jakovčević Z, Vasiljević M, Kumar R, Asrani RK. Effects of supplemented multicomponent mycotoxin detoxifying agent in laying hens fed aflatoxin B1 and T2-toxin contaminated feeds. Poult Sci (2023) 102: doi: 10.1016/j.psj.2023.102795
30. Riahi I, Ramos AJ, Raj J, Jakovčević Z, Farkaš H, Vasiljević M, Pérez-Vendrell AM. Effect of a mycotoxin binder (MMDA) on the growth performance, blood and carcass characteristics of broilers fed ochratoxin a and T-2 mycotoxin contaminated diets. Animals (2021) 11: doi: 10.3390/ani11113205
31. Raj J, Vasiljević M, Tassis P, Farkaš H, Männer K. Efficacy of a multicomponent mycotoxin detoxifying agent on concurrent exposure to zearalenone and T-2 mycotoxin in weaned pigs. Livest Sci (2020) 242:104295. doi:10.1016/j.livsci.2020.104295
32. Tsiouris V, Tassis P, Raj J, Mantzios T, Kiskinis K, Vasiljević M, Delić N, Petridou E, Brellou GD, Polizopoulou Z, et al. Investigation of a novel multicomponent mycotoxin detoxifying agent in amelioration of mycotoxicosis induced by aflatoxin-b1 and ochratoxin a in broiler chicks. Toxins (Basel) (2021) 13:1–18. doi:10.3390/toxins13060367




 Micotoxicosis prevention
Micotoxicosis prevention