L. Gámiz-Gracia1, N. Arroyo-Manzanares2, V. Rodríguez-Estévez3 and A.M. García-Campaña1
1Department of Analytical Chemistry, Faculty of Sciences, University of Granada.
2Department of Analytical Chemistry, Faculty of Chemistry, University of Murcia, Spain.
3Department of Animal Production, Faculty of Veterinary Medicine, University of Cordoba.
Mycotoxins are fungal toxins produced by several hundred species of molds that can grow on crops or food under certain conditions. The most important are those produced by molds of the genera Aspergillus, Fusarium and Penicillium.
 Mycotoxins have become one of the most reported contaminants worldwide, a fact that is reflected in the latest report from the EU Rapid Alert System for Food and Feed (RASFF)1. Mycotoxins have become one of the most reported contaminants worldwide, a fact that is reflected in the latest report from the EU Rapid Alert System for Food and Feed (RASFF)1. |
⇰ It should be noted that, despite the current state of knowledge and improvements in production and storage practices, it has not been possible to eradicate the development of fungi and molds and, therefore, the presence of mycotoxins in a large number of foods.
According to the Food and Agriculture Organization of the United Nations (FAO), it is estimated that 25% of cereal production and 20% of plant production worldwide are affected by mycotoxins, although recent studies consider that this figure may be underestimated2.
 ⇰ In addition, the effects of climate change are expected to increase the production of toxigenic fungi in the coming years3.
⇰ In addition, the effects of climate change are expected to increase the production of toxigenic fungi in the coming years3.
Despite the wide variety of known mycotoxins with various toxicological effects, the EU has set maximum permitted or recommended levels for only 14 of them (aflatoxins B1, B2, G1, G2 and M1, ochratoxin A, patulin, deoxynivalenol, zearalenone, fumonisins B1 and B2, T-2 and HT-2 toxins, citrinin) as well as for ergot sclerotia, in various food intended for human consumption4,5.
⇰ In the case of animal feed, the number of mycotoxins with maximum permitted or recommended levels is even lower, being limited to aflatoxin B1, ochratoxin A, deoxynivalenol, zearalenone, fumonisins B1 and B2, T-2 and HT-2 toxins, and ergot sclerotia6,7.

 Among farm animals, pigs are considered to be one of the most vulnerable species to the effects of mycotoxins. Among farm animals, pigs are considered to be one of the most vulnerable species to the effects of mycotoxins. |
⇰ Such effects may include changes in immune response (resulting in reduced vaccination efficacy), loss of appetite, abortions, agalactia, etc.
Table 1 shows a summary of the mycotoxins regulated in the European legislation for pig feed, their permitted or recommended limits, as well as their most common health effects.
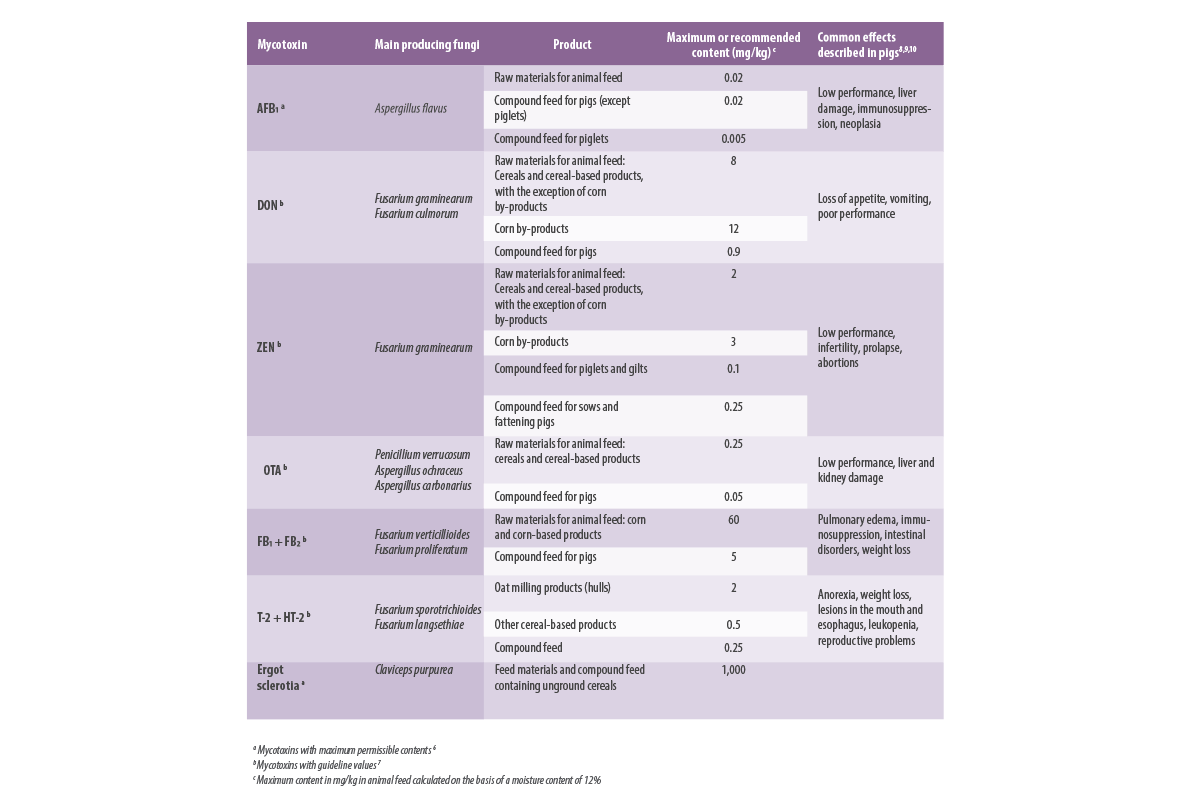
Table 1. Maximum permitted or recommended values in pig feed.
Ergot alkaloids, known since ancient times for their toxic effects, are not included in legislation (there is only a maximum content for ergot sclerotia, listed in Table 1), although they also pose a risk to livestock and it is expected that maximum contents for these compounds will be established in the near future.
Focusing on emerging mycotoxins







 Micotoxicosis prevention
Micotoxicosis prevention