Sabry El-khodary
Professor of Internal Medicine, and vice dean for post graduate studies and research affairs, Faculty of Veterinary Medicine, Mansoura University, Egypt
Numerous investigations have shown that, once ingested, mycotoxin-contaminated feed can induce harmful effects, such as hepatorenal toxicity, reproductive, cardiac, and neurotoxicity, as well as immunotoxicity in animals (Fang et al., 2022). When livestock ingests one or more mycotoxins, the effect on health can be acute, showing evident signs of disease or even causing death.![]() However, acute manifestation of mycotoxicosis is rare under farm conditions (Vieira et al., 2014).
However, acute manifestation of mycotoxicosis is rare under farm conditions (Vieira et al., 2014).
The effects of mycotoxin ingestion are mainly chronic, leading to hidden disorders with reduced ingestion, productivity, and fertility (Fink-Gremmels, 2008). Such effects cause severe economic losses due to: (Oswald et al., 2005; Storm et al., 2014a)

The immunotoxic effects of MYCOTOXINS in ruminants
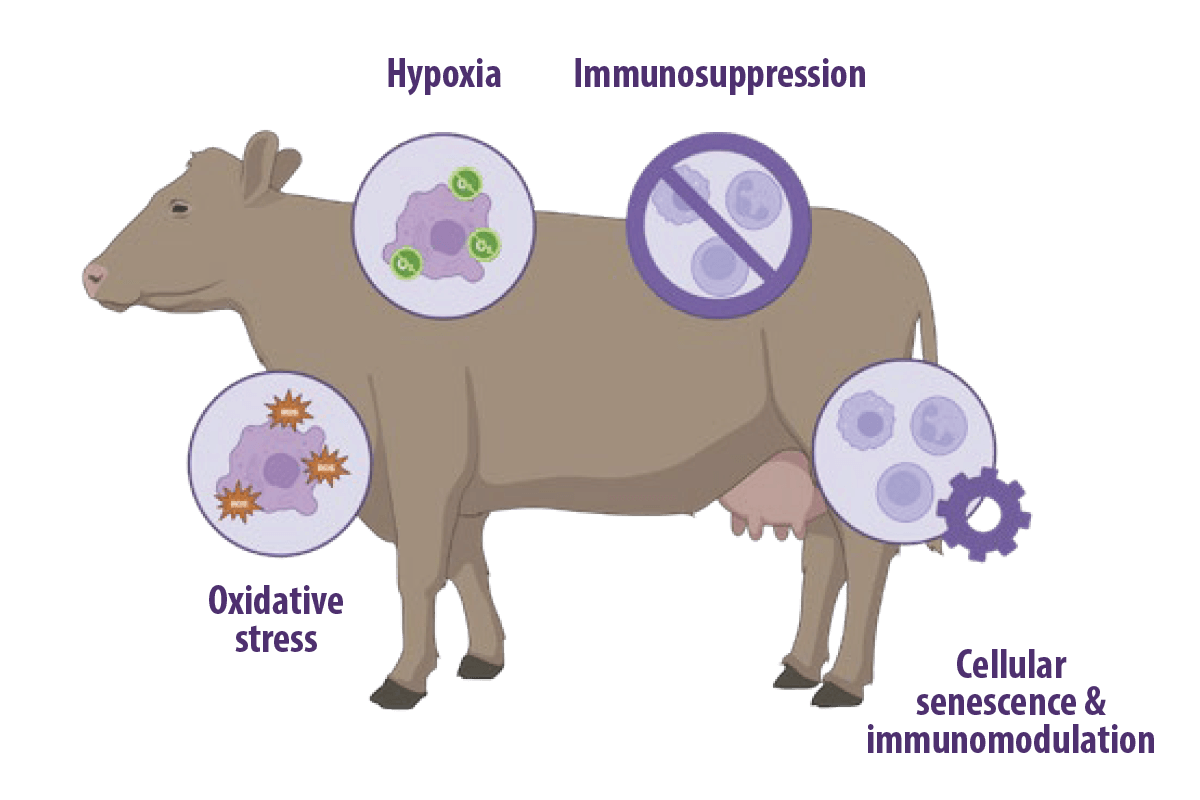
Mycotoxins have been proved to have immunosuppressive effects depending on: For example, aflatoxin B1 (AFB1) has immunotoxic consequences, such as the disruption of innate and acquired/adaptive immunity (Meissonnier et al., 2008; Mohsenzadeh et al., 2016). In ruminants, mycotoxins have been confirmed to induce oxidative stress, hypoxia, and immunosuppression. Additionally, emerging evidence shows that mycotoxins have the potential to induce cellular senescence, which is involved in their immunomodulatory effects (You et al., 2023).
![]() The mycotoxin(s) involved
The mycotoxin(s) involved![]() The concentration
The concentration![]() The parameter studied
The parameter studied
Penicillium-derived toxins P. roqueforti and P. paneum produce several secondary metabolites with immunosuppressive, antibacterial, and other not well-defined toxicological effects for animals (Storm et al., 2008; Storm et al., 2014b). Monascus ruber-derived toxins Based on in vitro results, immunotoxic effects of citrinin have been documented at very high doses (Stec et al., 2008).
Fusarium-derived toxins Fusarium-derived toxins, mainly deoxynivalenol (DON) and Fumonisin B1 (FB1), can drastically alter the defense mechanisms in the intestine, reducing epithelial integrity, cell proliferation and mucus production or increasing intestinal permeability, as well as the production of immunoglobulins and cytokines (Antonissen et al., 2014). For example, A. fumigatus produces several mycotoxins, including gliotoxin and tremorgens that are toxic to cattle. ⇰ Gliotoxin, an immune suppressant, has been present in animals infected with A. fumigatus (Bauer et al., 1989). In an insect model, the role of gliotoxin in increasing the virulence of A. fumigatus has been demonstrated A. fumigatus (Reeves et al., 2004). Several metabolites from Fusarium fungi, collectively known as Fusarium toxins, have shown deleterious effects in humans and livestock by targeting the immune and hepatic systems (Bondy and Pestka, 2000b; Trenholm et al., 1984).
The impact of mycotoxins on the ruminant immune system
In ruminants, AFB1, ochratoxin A (OTA), and DON are the three mycotoxins that have received the most scholarly attention and have been tested most routinely in clinical settings. These mycotoxins have been found not only to suppress immune responses but also to induce inflammation and even increase susceptibility to pathogens (Sun et al., 2023a). It is theorized that, with in the development of mycosis, mycotoxins produced by the invading fungi can suppress immunity, therefore increasing their infectivity (Whitlow and Hagler Jr, 2010).
Leukocytes and differential leukocytic count
The same authors added that the phagocytic capacity of granulocytes from all the steers was substantially reduced by week 5, whereas monocyte activity began to decline even earlier in the study. ⇰ Downregulated genes from treatment-fed steers indicates that iron utilization may be adversely affected by DON/ FUM exposure (Roberts et al., 2021b).![]() In a 3-week experimental case of mycotoxicosis in steers, there was leukocytosis with lymphocytosis, monocytosis, and neutropenia. However, 1.7 mg of DON and 3.5 mg of fumonisins (FUM) per kg of total ration were insufficient to cause significant cytotoxic effects on circulating mononuclear leukocytes in these animals (Roberts et al., 2021b).
In a 3-week experimental case of mycotoxicosis in steers, there was leukocytosis with lymphocytosis, monocytosis, and neutropenia. However, 1.7 mg of DON and 3.5 mg of fumonisins (FUM) per kg of total ration were insufficient to cause significant cytotoxic effects on circulating mononuclear leukocytes in these animals (Roberts et al., 2021b).![]() In a study with control-fed steers, CD8+ lymphocytes decreased during the first two weeks of the treatment period, while treatment-fed animals (DON and FUM) had significantly higher CD4-CD8+ lymphocyte populations during week 2 (Roberts et al., 2021b).
In a study with control-fed steers, CD8+ lymphocytes decreased during the first two weeks of the treatment period, while treatment-fed animals (DON and FUM) had significantly higher CD4-CD8+ lymphocyte populations during week 2 (Roberts et al., 2021b).![]() It has been concluded that bovine cells are more sensitive to DON than those from other livestock species (Novak et al., 2018). However, their effect on body condition or growth status was not been formally evaluated (Pestka, 2008).
It has been concluded that bovine cells are more sensitive to DON than those from other livestock species (Novak et al., 2018). However, their effect on body condition or growth status was not been formally evaluated (Pestka, 2008).![]() It has been suggested that the CD4:CD8 ratio could be a candidate biomarker of early DON exposure in beef cattle, which may be attenuated by co-occurring fumonisin toxicity (Taranu et al., 2010).
It has been suggested that the CD4:CD8 ratio could be a candidate biomarker of early DON exposure in beef cattle, which may be attenuated by co-occurring fumonisin toxicity (Taranu et al., 2010).![]() RNA-Seq analysis has revealed 217 differentially expressed genes between treatment- and control-fed steers, with mycotoxin exposure broadly leading to down-regulation of annotated features.
RNA-Seq analysis has revealed 217 differentially expressed genes between treatment- and control-fed steers, with mycotoxin exposure broadly leading to down-regulation of annotated features.![]() Previous studies have shown alterations in cellular oxygen transport mechanisms by in vitro administration of fumonisins (Osweiler et al., 1993; Yin et al., 1996).
Previous studies have shown alterations in cellular oxygen transport mechanisms by in vitro administration of fumonisins (Osweiler et al., 1993; Yin et al., 1996).![]() In an experimental study, mycotoxins have shown to reduce phagocytic activity in finishing-stage steers, regardless of prior mycotoxin exposure (Roberts et al., 2021a).
In an experimental study, mycotoxins have shown to reduce phagocytic activity in finishing-stage steers, regardless of prior mycotoxin exposure (Roberts et al., 2021a).
Inflammatory Response, Neutrophil Chemotaxis and Immunoglobulins
The same authors found that early mycotoxin exposure was accompanied with a numerical increase in neutrophil abundance, but at the same time, functional assays showed a slight decrease in granulocyte phagocytosis and oxidative burst. Additionally, immunoglobulin-related pathways were enriched in animals exposed to DON/FUM on day 21.![]() In an experimental study in finishing steers, exposure to DON/FUM induced upregulation of inflammatory response times at days 7 and 21, along with neutrophil chemotaxis times at day 7 (Roberts et al., 2021a).
In an experimental study in finishing steers, exposure to DON/FUM induced upregulation of inflammatory response times at days 7 and 21, along with neutrophil chemotaxis times at day 7 (Roberts et al., 2021a).![]() The effects of DON on laboratory animal immunoglobulin levels have been previously reviewed (Bondy and Pestka, 2000a), showing alterations in immunoglobulin production in a class-dependent manner. However, disruptions to antibody production are an important nonlinear toxicological outcome in cattle (Dänicke et al., 2018).
The effects of DON on laboratory animal immunoglobulin levels have been previously reviewed (Bondy and Pestka, 2000a), showing alterations in immunoglobulin production in a class-dependent manner. However, disruptions to antibody production are an important nonlinear toxicological outcome in cattle (Dänicke et al., 2018).
Specifically, AFB1 increases the synthesis of IFN-gamma, a Th1 cytokine involved in the cell mediated immune response, and decreases IL-4 synthesis, a Th2 cytokine involved in the humoral response (Pierron et al., 2016).
![]() DIA and DAVID analysis tools revealed the effects of dietary mycotoxin treatments on the beef cattle transcriptome, showing consistent inhibition of focal adhesion, ECM signaling and PI3K-Akt signaling pathways (Roberts et al., 2021a).
DIA and DAVID analysis tools revealed the effects of dietary mycotoxin treatments on the beef cattle transcriptome, showing consistent inhibition of focal adhesion, ECM signaling and PI3K-Akt signaling pathways (Roberts et al., 2021a).![]() In vitro studies have demonstrated a suppressive effect of aflatoxins (AF) on inflammatory cytokine levels in cattle (Kurtz and Czuprynski, 1992).
In vitro studies have demonstrated a suppressive effect of aflatoxins (AF) on inflammatory cytokine levels in cattle (Kurtz and Czuprynski, 1992).![]() In pigs, mycotoxin exposure inhibits lymphocyte proliferation and alters cytokine production.
In pigs, mycotoxin exposure inhibits lymphocyte proliferation and alters cytokine production.
DON, nivalenol (NIV), zearalenone (ZEN), and FB1 have been proven to modulate the secretory mucins MUC5AC and MUC5B, as well as total mucin-like glycoprotein secretion, all of which are essential components of host mucosal immunity (Wan et al., 2014).![]() Significant changes in some coagulation factors in the extrinsic pathway in the intoxicated lambs and prothrombin time determination could be used as an indicator of aflatoxicosis in lambs (Fernández et al., 1995).
Significant changes in some coagulation factors in the extrinsic pathway in the intoxicated lambs and prothrombin time determination could be used as an indicator of aflatoxicosis in lambs (Fernández et al., 1995).
Susceptibility to infection and failure of vaccination Mycotoxin exposure can affect the infection severity of some pathogens, including bacteria, viruses, and parasites. Their specific mechanisms of action include three aspects: Mycotoxin-associated increased susceptibility to infection has been documented in experimental and natural conditions.
⇰ The presence of mycotoxins in the feed may lead to a decline in vaccinal immunity and to the occurrence of disease even in properly vaccinated flocks (Pierron et al., 2016). In other studies, cattle exposed to mycotoxin mixtures were colonized by two or more STECs, suggesting that mycotoxins facilitate co-infection (Baines et al., 2011a; Baines et al., 2011b). In lambs, experimental aflatoxicosis revealed that albumin and alfa-globulin levels were lower for intoxicated lambs than in the control group. Although beta-globulin concentrations did not change, increases in gamma-globulins levels in dosed lambs were observed throughout the experiment. ⇰ These results suggest that AF cause a failure in the lambs’ acquired immunity by decreasing antibody production and altering serum proteins (Fernández et al., 1997). However, in another experiment, results indicate that a decline in the average daily gain was the most sensitive indicator of aflatoxicosis in lambs, showing that the altered immune response could render the animals more susceptible to infectious diseases (Fernández et al., 2000).![]() In calves, exposure to AFs has shown to increase susceptibility to Shiga toxin-producing Escherichia coli (STEC) (Baines et al., 2013a).
In calves, exposure to AFs has shown to increase susceptibility to Shiga toxin-producing Escherichia coli (STEC) (Baines et al., 2013a).![]() Alteration of lymphocyte proliferation and cytokine production may explain vaccine failures that have been observed in vivo.
Alteration of lymphocyte proliferation and cytokine production may explain vaccine failures that have been observed in vivo.![]() Synergistic effects between viruses and mycotoxins have been extensively studied in swine (Gan et al., 2022; Gan et al., 2018) and the outcomes of the ingestion of mycotoxin-contaminated feed are increased susceptibility to infectious diseases, reactivation of chronic infection and a decreased vaccine efficacy (Pierron et al., 2016).
Synergistic effects between viruses and mycotoxins have been extensively studied in swine (Gan et al., 2022; Gan et al., 2018) and the outcomes of the ingestion of mycotoxin-contaminated feed are increased susceptibility to infectious diseases, reactivation of chronic infection and a decreased vaccine efficacy (Pierron et al., 2016).![]() Exposing calves to 1–3 ppb of AF and 50–350 ppb of FUM facilitated STEC-associated outbreaks. In addition, in vitro inclusion of 0.02 ppb of AF in the growth media of STECs resulted in higher cytotoxin production and cytotoxicity, highlighting the role of mycotoxins in STEC pathogenesis (Baines et al., 2013b).
Exposing calves to 1–3 ppb of AF and 50–350 ppb of FUM facilitated STEC-associated outbreaks. In addition, in vitro inclusion of 0.02 ppb of AF in the growth media of STECs resulted in higher cytotoxin production and cytotoxicity, highlighting the role of mycotoxins in STEC pathogenesis (Baines et al., 2013b).
![]() An experiment carried out by Dzidic et al. (2010) indicated that sheep fed 300 mg of mycophenolic acid/sheep/day from contaminated silage did not show any immunodepression effects
An experiment carried out by Dzidic et al. (2010) indicated that sheep fed 300 mg of mycophenolic acid/sheep/day from contaminated silage did not show any immunodepression effects
Although mycotoxins have been confirmed to modulate the effects of bacteria within animals and increase their virulence, it has also been suggested that strains of L. acidophilus CIP 76.13T and L. delbrueckii subsp. bulgaricus CIP 101027T may be included in feed to reduce mycotoxin contamination (Ragoubi et al., 2021).![]() As mycotoxin exposure can be detrimental to certain intestinal microbial populations, the use of beneficial bacteria can alleviate these effects. For example, Lactobacillus is a critical genus for detoxifying OTA in vivo (Guerre, 2020; Jin et al., 2021; Sun et al., 2023b).
As mycotoxin exposure can be detrimental to certain intestinal microbial populations, the use of beneficial bacteria can alleviate these effects. For example, Lactobacillus is a critical genus for detoxifying OTA in vivo (Guerre, 2020; Jin et al., 2021; Sun et al., 2023b).
Antonissen, G., Martel, A., Pasmans, F., Ducatelle, R., Verbrugghe, E., Vandenbroucke, V., Li, S., Haesebrouck, F., Van Immerseel, F.,Croubels, S., 2014. The impact of Fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins 6, 430-452.Baines, D., Erb, S., Lowe, R., Turkington, K., Sabau, E., Kuldau, G., Juba, J., Masson, L., Mazza, A., Roberts, R., 2011a. A prebiotic, Celmanax™, decreases Escherichia coli O157: H7 colonization of bovine cells and feed-associated cytotoxicity in vitro. BMC research notes 4, 1-13. Baines, D., Erb, S., Turkington, K., Kuldau, G., Juba, J., Masson, L., Mazza, A., Roberts, R., 2011b. Mouldy feed, mycotoxins and Shiga toxin-producing Escherichia colicolonization associated with Jejunal Hemorrhage Syndrome in beef cattle. BMC Veterinary Research 7, 1-8. Baines, D., Sumarah, M., Kuldau, G., Juba, J., Mazza, A., Masson, L., 2013a. Aflatoxin, fumonisin and Shiga toxin-producing Escherichia coli infections in calves and the effectiveness of Celmanax®/Dairyman’s Choice™ applications to eliminate morbidity and mortality losses.Toxins (Basel) 5, 1872-1895. Baines, D., Sumarah, M., Kuldau, G., Juba, J., Mazza, A., Masson, L., 2013b. Aflatoxin, fumonisin and shiga toxin-producing Escherichia coli infections in calves and the effectiveness of Celmanax®/Dairyman’s Choice™ applications to eliminate morbidity and mortality losses.Toxins 5, 1872-1895. Bauer, J., Gareis, M., Bott, A., Gedek, B., 1989. Isolation of a mycotoxin (gliotoxin) from a bovine udder infected with Aspergillusfumigatus. Journal of Medical and Veterinary Mycology 27, 45-50. Bondy, G.S., Pestka, J.J., 2000a. Immunomodulation by fungal toxins. Journal of Toxicology and Environmental Health Part B: CriticalReviews 3, 109-143. Bondy, G.S., Pestka, J.J., 2000b. Immunomodulation by fungal toxins. J Toxicol Environ Health B Crit Rev 3, 109-143. Dänicke, S., Winkler, J., Meyer, U., Kersten, S., Wernike, K., Beer, M., Frahm, J., 2018. Antibody response of growing German Holstein bulls to a vaccination against bovine viral diarrhea virus (BVDV) is influenced by Fusarium toxin exposure in a non-linear fashion. Mycotoxin research 34, 123-139. Dzidic, A., Meyer, H.H., Bauer, J., Pfaffl, M.W., 2010. Long-term effects of mycophenolic acid on the immunoglobulin and inflammatory marker-gene expression in sheep white blood cells. Mycotoxin research 26, 235-240.Fang, M., Hu, W., Liu, B., 2022. Protective and detoxifying effects conferred by selenium against mycotoxins and livestock viruses: A review. Front Vet Sci 9, 956814. Fernandez, A., Hernandez, M., Sanz, M.C., Verde, M.T., Ramos, J.J., 1997. Serological serum protein fraction and responses to Brucellamelitensis in lambs fed aflatoxins. Vet Hum Toxicol 39, 137-140. Fernández, A., Hernández, M., Verde, M.T., Sanz, M., 2000. Effect of aflatoxin on performance, hematology, and clinical immunology in lambs. Can J Vet Res 64, 53-58. Fernández, A., Ramos, J.J., Saez, T., Sanz, M.C., Verde, M.T., 1995. Changes in the coagulation profile of lambs intoxicated with aflatoxin in their feed. Vet Res 26, 180-184. Fink-Gremmels, J., 2008. The role of mycotoxins in the health and performance of dairy cows. Vet J 176, 84-92. Gan, F., Hou, L., Xu, H., Liu, Y., Chen, X., Huang, K., 2022. PCV2 infection aggravates OTA-induced immunotoxicity in vivo and in vitro. Ecotoxicology and Environmental Safety 235, 113447. Gan, F., Zhou, Y., Qian, G., Huang, D., Hou, L., Liu, D., Chen, X., Wang, T., Jiang, P., Lei, X., 2018. PCV2 infection aggravates ochratoxinA-induced nephrotoxicity via autophagy involving p38 signaling pathway in vivo and in vitro. Environmental Pollution 238, 656-662. Guerre, P., 2020. Mycotoxin and gut microbiota interactions. Toxins 12, 769.Jin, J., Beekmann, K., Ringø, E., Rietjens, I.M., Xing, F., 2021. Interaction between food-borne mycotoxins and gut microbiota: A review. Food Control 126, 107998. Kurtz, R.S., Czuprynski, C.J., 1992. Effect of aflatoxin B1 on in vitro production of interleukin-1 by bovine mononuclear phagocytes.Veterinary immunology and immunopathology 34, 149-158. Meissonnier, G.M., Pinton, P., Laffitte, J., Cossalter, A.-M., Gong, Y.Y., Wild, C.P., Bertin, G., Galtier, P., Oswald, I.P., 2008. Immunotoxicity of aflatoxin B1: impairment of the cell-mediated response to vaccine antigen and modulation of cytokine expression. Toxicology and applied pharmacology 231, 142-149. Mohsenzadeh, M.S., Hedayati, N., Riahi-Zanjani, B., Karimi, G., 2016. Immunosuppression following dietary aflatoxin B1 exposure: a review of the existing evidence. Toxin Reviews 35, 121-127. Novak, B., Vatzia, E., Springler, A., Pierron, A., Gerner, W., Reisinger, N., Hessenberger, S., Schatzmayr, G., Mayer, E., 2018. Bovine peripheral blood mononuclear cells are more sensitive to deoxynivalenol than those derived from poultry and swine. Toxins 10, 152. Oh, S.-Y., Balch, C.G., Cliff, R.L., Sharma, B.S., Boermans, H.J., Swamy, H., Quinton, V.M., Karrow, N.A., 2013. Exposure to Penicillium mycotoxins alters gene expression of enzymes involved in the epigenetic regulation of bovine macrophages (BoMacs). Mycotoxin research29, 235-243. Oswald, I.P., Marin, D.E., Bouhet, S., Pinton, P., Taranu, I., Accensi, F., 2005. Immunotoxicological risk of mycotoxins for domestic animals. Food Addit Contam 22, 354-360. Osweiler, G., Kehrli, M., Stabel, J., Thurston, J., Ross, P., Wilson, T., 1993. Effects of fumonisin-contaminated corn screenings on growth and health of feeder calves. Journal of animal science 71, 459-466. Pestka, J.J., 2008. Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food additives and contaminants 25,1128-1140. Pierron, A., Alassane-Kpembi, I., Oswald, I.P., 2016. Impact of mycotoxin on immune response and consequences for pig health. AnimNutr 2, 63-68. Ragoubi, C., Quintieri, L., Greco, D., Mehrez, A., Maatouk, I., D’Ascanio, V., Landoulsi, A., Avantaggiato, G., 2021. Mycotoxin Removal by Lactobacillus spp. and Their Application in Animal Liquid Feed. Toxins (Basel) 13. Reeves, E.P., Messina, C., Doyle, S., Kavanagh, K., 2004. Correlation between gliotoxin production and virulence of Aspergillus fumigatusin Galleria mellonella. Mycopathologia 158, 73-79. Roberts, H.L., Bionaz, M., Jiang, D., Doupovec, B., Faas, J., Estill, C.T., Schatzmayr, D., Duringer, J.M., 2021a. Effects of deoxynivalenol and fumonisins fed in combination to beef cattle: Immunotoxicity and gene expression. Toxins 13, 714. Roberts, H.L., Bionaz, M., Jiang, D., Doupovec, B., Faas, J., Estill, C.T., Schatzmayr, D., Duringer, J.M., 2021b. Effects of Deoxynivalenol and Fumonisins Fed in Combination to Beef Cattle: Immunotoxicity and Gene Expression. Toxins (Basel) 13. Stec, J., Rachubik, J., Szczotka, M., Kuźmak, J., 2008. Effects of penicillium mycotoxins: citrinin, ochratoxin A, and patulin on in vitro proliferation of bovine lymphocytes. Bull. Vet. Inst. Pulawy 52, 163-167.Storm, I., Sørensen, J.L., Rasmussen, R.R., Nielsen, K.F., Thrane, U., 2008. Mycotoxins in silage. Stewart Postharvest Rev 4, 1-12. Storm, I.M., Rasmussen, R.R., Rasmussen, P.H., 2014a. Occurrence of pre- and post-harvest mycotoxins and other secondary metabolites in Danish maize silage. Toxins (Basel) 6, 2256-2269.Storm, I.M.D., Rasmussen, R.R., Rasmussen, P.H., 2014b. Occurrence of pre-and post-harvest mycotoxins and other secondary metabolites in Danish maize silage. Toxins 6, 2256-2269. Sun, Y., Song, Y., Long, M., Yang, S., 2023a. Immunotoxicity of Three Environmental Mycotoxins and Their Risks of Increasing Pathogen Infections. Toxins (Basel) 15.Sun, Y., Song, Y., Long, M., Yang, S., 2023b. Immunotoxicity of Three Environmental Mycotoxins and Their Risks of Increasing Pathogen Infections. Toxins 15, 187. Taranu, I., Marin, D.E., Burlacu, R., Pinton, P., Damian, V., Oswald, I.P., 2010. Comparative aspects of in vitro proliferation of human and porcine lymphocytes exposed to mycotoxins. Archives of Animal Nutrition 64, 383-393. Trenholm, H.L., Hamilton, R.M., Friend, D.W., Thompson, B.K., Hartin, K.E., 1984. Feeding trials with vomitoxin (deoxynivalenol)-contaminated wheat: effects on swine, poultry, and dairy cattle. J Am Vet Med Assoc 185, 527-531. Vieira, M.L., Johann, S., Hughes, F.M., Rosa, C.A., Rosa, L.H., 2014. The diversity and antimicrobial activity of endophytic fungi associated with medicinal plant Baccharis trimera (Asteraceae) from the Brazilian savannah. Can J Microbiol 60, 847-856. Wan, L.Y., Allen, K.J., Turner, P.C., El-Nezami, H., 2014. Modulation of mucin mRNA (MUC5AC and MUC5B) expression and protein production and secretion in Caco-2/HT29-MTX co-cultures following exposure to individual and combined Fusarium mycotoxins. ToxicolSci 139, 83-98. Whitlow, L., Hagler Jr, W., 2010. Mycotoxin effects in dairy cattle. Mid-South Ruminant.Yin, J.-J., Smith, M.J., Eppley, R.M., Page, S.W., Sphon, J.A., 1996. Effects of Fumonisin B1on Oxygen Transport in Membranes. Biochemical and biophysical research communications 225, 250-255. You, L., Nepovimova, E., Valko, M., Wu, Q., Kuca, K., 2023. Mycotoxins and cellular senescence: the impact of oxidative stress, hypoxia, and immunosuppression. Arch Toxicol 97, 393-404.
![]() BIBLIOGRAPHY
BIBLIOGRAPHY




 Micotoxicosis prevention
Micotoxicosis prevention