Mursal Abdulkadir Hersi1, Dogukan Kaya2 and Ercument Genc1*
1Department of Fisheries and Aquaculture Engineering, Faculty of Agriculture, Ankara University, Ankara, Turkey
2Agricultural Applications and Research Center, Tokat Gaziosmanpasa University, Tokat, Turkey
Corresponding author: [email protected]
In the first part of this article, we reviewed the main effects associated with mycotoxin exposure in aquatic species. In the second part of this article, the various mycotoxin adsorbing agents and feed additives used in aquaculture and their efficacy are reviewed and discussed.
Mycotoxin adsorbing agents and detoxifying feed additives
![]() The main goal of aquaculture and other food and feed production industries is to prevent mycotoxin occurrence. However, the formation of mycotoxins is sometimes inevitable due to many environmental factors and microbial interactions.
The main goal of aquaculture and other food and feed production industries is to prevent mycotoxin occurrence. However, the formation of mycotoxins is sometimes inevitable due to many environmental factors and microbial interactions.
In such cases, substances, compounds, or microbes can:
- ⇰ Remove mycotoxins by excretion from the body of exposed animals.
- ⇰ Minimize their adsorption by the animal
- ⇰ Mitigate their effects
(Vila-Donat et al., 2018)
 Nevertheless, applying such substances does not necessarily mean that animals should be fed with feed containing mycotoxins over the maximum allowable doses.
Nevertheless, applying such substances does not necessarily mean that animals should be fed with feed containing mycotoxins over the maximum allowable doses.Currently, three basic methods (chemical, physical and biological) can be used to detoxify mycotoxins, all of which have undergone substantial research studies (Karlovsky et al., 2016; Zhou et al., 2018).

 CHEMICAL METHODS
CHEMICAL METHODS
Chemical approaches imply the application of different chemical compounds such as oxidizing substances, alkalis, and acids to destroy mycotoxins (Čolović et al. 2019).
However, the use of chemicals is associated with severe health effects. For example:
 Chlorine dioxide used to purify mycotoxin-contaminated feed has been reported to deteriorate both the taste and texture of diets.
Chlorine dioxide used to purify mycotoxin-contaminated feed has been reported to deteriorate both the taste and texture of diets. Ozone, which can also destroy most mycotoxins, is unsafe for animals and humans.
Ozone, which can also destroy most mycotoxins, is unsafe for animals and humans. Sodium hypochlorite, which is been reported to disinfect trichothecenes, has detrimental consequences (Yu et al., 2020).
Sodium hypochlorite, which is been reported to disinfect trichothecenes, has detrimental consequences (Yu et al., 2020).
Therefore, applying chemicals to detoxify mycotoxins is not recommended (Conte et al., 2020).
![]() PHYSICAL METHODS
PHYSICAL METHODS
Physical methods include detoxifying mycotoxins through adsorption (Yu et al., 2020). After ingestion and before the mycotoxin is absorbed and systematically circulated, binding or adsorbing agents bind to it so it can be easily excreted.
Mycotoxin adsorbents have different mechanisms to bind to mycotoxins such as hydrophobic interactions, dipole-dipole interactions, and electrostatic attraction or repulsion (Di Gregorio et al., 2014).
⇰ The success of these sequestering or adsorbing agents are also affected by their physical properties, such as their shape and pores, the polarity and solubility of the targeted mycotoxin, and the pH level (Kabak et al., 2006).
Although mycotoxin binders are effective methods compared to chemical mechanisms, they cannot detoxify all toxins (Battilani, 2008; Ji y Xie, 2021).

![]() BIOLOGICAL METHODS
BIOLOGICAL METHODS
With biological methods, mycotoxins are not removed from the body but rather bio-transformed into less toxic compounds. This mechanism involves the use of microorganisms (bacteria, fungi, etc.) and enzymes to break down mycotoxins (Wielogórska et al., 2016).

However, there are many challenges in the application of biological methods, including:
- ⇰ Limited data on the mode of action of microbes during mycotoxin detoxification.
- ⇰ The safety of derived products after degradation.
- ⇰ How the process can affect the nutrient content of feeds.
(Conte et al., 2020)
Nevertheless, under favorable environmental conditions, the microbes involved have a higher detoxification capacity and, thus, this strategy is considered the best remedy against mycotoxins (Zychowski et al. 2013).
Mycotoxin Binders
As mentioned above, different adsorbing agents or nano-clay particles are used to remove mycotoxins from the body of the exposed animal.
 Due to their importance and wide range of applications in recent years, nano-clay particles have been significantly incorporated into various fields, including aquaculture, to improve water quality parameters (Nathanail et al., 2016).
Due to their importance and wide range of applications in recent years, nano-clay particles have been significantly incorporated into various fields, including aquaculture, to improve water quality parameters (Nathanail et al., 2016).
Adsorbing agents are ultrafine nanoparticles that are usually smaller than 100 nm and have the potential to provide a good surface for mycotoxin binding (Feng et al., 2009). They can be classified as synthetic adsorbents, obtained through mechanical or machine grinding, and natural adsorbents (Wagner et al., 2014).
The most common nanoparticles for mycotoxin binding are aluminosilicates (hydrated sodium calcium aluminosilicate, bentonites, zeolites, etc.) and yeast cell walls (Arana et al., 2011).
![]() The mycotoxin removal efficiency rate of most nanoparticles or adsorbing agents is estimated to be between 20% and 80% (Nathanail et al., 2016).
The mycotoxin removal efficiency rate of most nanoparticles or adsorbing agents is estimated to be between 20% and 80% (Nathanail et al., 2016).
However, one major drawback of mycotoxin binders is their ability to also adsorb other micronutrients in the animals’ bodies which may result in mineral deficiency (Kolosova y Stroka, 2011).
Aluminosilicates
Representing the most abundant rock-forming minerals, aluminosilicates characterize the silicon-oxygen tetrahedron bonded together by octahedral aluminum sheets (Tapia-Salazar et al., 2010). Los silicatos de lámina (filosilicatos) y los tectosilicatos son los dos grupos principales de aluminosilicatos.
Among the most common adsorbents in the phyllosilicates group are bentonites, kaolinites, smectites, and montmorillonites, while zeolites are the most widely used sequestering agents in the tectosilicates group (Di Gregorio et al., 2014).
All these mycotoxin binders deliver a large surface area for binding mycotoxins and are affected by their size, porous, shape, and selectivity (Huwig et al. 2001).

BENTONITES
Bentonites, also known as montmorillonites, are a group of sheet silicate clay particles that are efficient in mycotoxin adsorptions (Kolosova y Stroka, 2011).
⇰ The binding capacity of bentonites is associated with the presence and content of smectite minerals (montmorillonite), the primary raw material used in producing nano-clay particles (Benvindo da Luz y Lins, 2008).
 Bentonites are cosmopolitan minerals and are usually incorporated into most commercially available aquafeeds to improve water quality indices (Hussain 2018).
Bentonites are cosmopolitan minerals and are usually incorporated into most commercially available aquafeeds to improve water quality indices (Hussain 2018).
In recent years, many studies have been conducted to investigate the role of bentonite on mycotoxin detoxification in aquaculture.
![]() A survey by Neeratanaphan y Tengjaroenkul (2018) evaluated the efficacy of 1% sodium bentonite in improving the growth and tissue histomorphology of Nile tilapia fed with diets containing 10-40 mg/kg of AFB1.
A survey by Neeratanaphan y Tengjaroenkul (2018) evaluated the efficacy of 1% sodium bentonite in improving the growth and tissue histomorphology of Nile tilapia fed with diets containing 10-40 mg/kg of AFB1.
Pathological examinations of the groups fed with diets containing aflatoxin without bentonite supplementation showed significantly decreased growth rate, lesions in the gills, leukocyte infiltration, vascular dilation, degeneration, and lack of integrity in the liver.
⇰ SHowever, this study detected improvements in growth performance and tissue histomorphology when 1% sodium bentonite was supplemented with tilapia feeds.
![]() Similar results were reported by Hussain et al. (2017b) when the amelioration effect of 0.5% calcium bentonite clay against growth retardation caused by 2 and 4 mg/ kg of aflatoxins was examined.
Similar results were reported by Hussain et al. (2017b) when the amelioration effect of 0.5% calcium bentonite clay against growth retardation caused by 2 and 4 mg/ kg of aflatoxins was examined.
⇰ Before the supplementation of bentonite, the animals experienced significant growth reductions and poor feed utilization. Still, considerable growth and feed efficiency improvements were noted when feed containing adsorbent was fed to animals.
![]() Bentonites are mainly used for aflatoxin detoxification.
Bentonites are mainly used for aflatoxin detoxification.
![]() Kannewischer (2006) emphasized that the application of bentonites in aquaculture has a promising result and, therefore, should be used for detoxification.
Kannewischer (2006) emphasized that the application of bentonites in aquaculture has a promising result and, therefore, should be used for detoxification.

HYDRATED SODIUM CALCIUM ALUMINOSILICATE
Hydrated sodium calcium aluminosilicate is another potent adsorbent of mycotoxins in general, particularly aflatoxins (Di Gregorio et al. 2014).
⇰ The agent, generally used as an anti-caking agent, has also demonstrated its importance in mycotoxin binding and removal from the body.
![]() Huwig et al. (2001) discussed the efficacy of different mycotoxin binders, including zeolites, bentonites, yeast cell walls, and hydrated sodium calcium aluminosilicate.
Huwig et al. (2001) discussed the efficacy of different mycotoxin binders, including zeolites, bentonites, yeast cell walls, and hydrated sodium calcium aluminosilicate.
They concluded that hydrated sodium calcium aluminosilicate had the highest mycotoxin removal capacity.

![]() Fadl et al. (2020) evaluated the amelioration effects of dietary hydrated sodium aluminium silicates (0.2-0.5g) against the toxicity of ochratoxin A (1 mg/kg) on Nile tilapia growth, serum biochemical and tissue histopathology for six weeks.
Fadl et al. (2020) evaluated the amelioration effects of dietary hydrated sodium aluminium silicates (0.2-0.5g) against the toxicity of ochratoxin A (1 mg/kg) on Nile tilapia growth, serum biochemical and tissue histopathology for six weeks.
⇰ The results showed that the supplementation of hydrated sodium aluminium silicates protected from abnormal histopathological effects, maintained stable growth performance, and promoted healthy biochemical parameters.
![]() Likewise, El-Alim et al. (2017) also tested the efficacy of 0.5% hydrated sodium aluminum silicate supplementation against toxicity induced by 2.5 mg/kg of aflatoxins on Nile tilapia for six weeks.
Likewise, El-Alim et al. (2017) also tested the efficacy of 0.5% hydrated sodium aluminum silicate supplementation against toxicity induced by 2.5 mg/kg of aflatoxins on Nile tilapia for six weeks.
⇰ Anemia, leukopenia, lymphopenia, decreased phagocytic index, and a mortality rate of more than 16% in the groups fed diets containing aflatoxin alone were detected.
⇰ However, improvement in growth and biochemical parameters was reported in groups fed diets containing both aflatoxin and hydrated sodium aluminium silicate.
ZEOLITES
Zeolites are microporous tectosilicate crystalline aluminosilicates used extensively in aquaculture in recent years.
⇰ Due to their outstanding physical and chemical properties, including adsorption and ion exchange, various industries have applied zeolites for different purposes (Ghasemi et al., 2018).
|
![]() A three-month experiment carried out by Hassaan et al. (2020) revealed that aflatoxins at a dose of 3 mg/kg caused a significant decrease in feed use, growth rate, digestion, and antioxidant enzymes and increased liver enzymes, as well as aggravated DNA and left residue in the body of Nile tilapia.
A three-month experiment carried out by Hassaan et al. (2020) revealed that aflatoxins at a dose of 3 mg/kg caused a significant decrease in feed use, growth rate, digestion, and antioxidant enzymes and increased liver enzymes, as well as aggravated DNA and left residue in the body of Nile tilapia.
⇰ However, during this study, most of these pathological results were reduced by adding 5-10 g/kg nano-zeolite particles to tilapia feed.
![]() Different authors have reported similar results using other fish species such as Nile tilapia (Zahran et al., 2020) and rainbow trout (Alinezhad et al., 2020).
Different authors have reported similar results using other fish species such as Nile tilapia (Zahran et al., 2020) and rainbow trout (Alinezhad et al., 2020).


ACTIVATED CHARCOAL
Activated charcoal is a substance produced after biomass undergoes a process called pyrolysis. The agent is highly porous and is one of the most efficient mycotoxin adsorbents mainly used in the livestock industry (Avantaggiato et al., 2005). In addition to binding mycotoxins, in aquaculture, activated charcoal is used for different purposes, such as water purification and improving fish welfare (Selim et al., 2013).
⇰ The capacity of activated charcoal to bind or sequester mycotoxins is affected by its size, shape, porosity, and structure. Like other adsorbents, activated charcoal tends to attach to other micronutrients in the body, especially when they are higher in presence than mycotoxins, hence should be addressed (Vekiru et al. 2007).
Many studies have shown the positive effect of activated charcoal in mitigating mycotoxins, especially vomitoxin (Diaz y Smith 2005).
 Selim et al. (2013) revealed that aflatoxins significantly damage growth and blood biochemistry parameters of Nile tilapia, but the addition of activated charcoal to the feed results in improvements in haematological and growth performance indices.
Selim et al. (2013) revealed that aflatoxins significantly damage growth and blood biochemistry parameters of Nile tilapia, but the addition of activated charcoal to the feed results in improvements in haematological and growth performance indices.
Microorganisms and other feed additives
MICROBES
In addition to the application of mineral-binding materials for mycotoxins, microorganisms that adsorb or decompose the toxic substances are also applied.
MYCOTOXIN ADSORPTION
For adsorption, various microbes have a unique cell wall structure that can bind with different mycotoxins through different interactions such as hydrogen bonds and ionic and hydrophobic bonding (Haskard et al., 2001).
⇰ This is achieved before mycotoxin interaction with the body and significantly reduces mycotoxin bioavailability (Kolosova y Stroka, 2011).
The most commonly used microbes for this purpose are Saccharomyces cerevisiae and lactic acid bacteria.
SACCHAROMYCES CEREVISIAE
S. cerevisiae has a variety of applications in both the food and feed industry and has long been used in fermentation processes (Taheur et al., 2017).
Authors have reported that S. cerevisiae adsorbs and removes different mycotoxins (Zearalenone, Ochratoxin A, and Fumonisins) from the bodies of animals (Fruhauf et al., 2012; Taheur et al., 2017).
LACTIC ACID BACTERIA
Lactic acid bacteria are gram-positive bacterial groups commonly found in dairy products and decaying plant biomass.
Previously, different species of this group, such as Lactobacillus rhamnosus, have shown a strong ability to sequester mycotoxins, particularly aflatoxins and Zearalenone (Rahaie et al., 2012).
MYCOTOXIN DETOXIFICATION
Microorganisms may also, at some point in their life cycle, secrete chemicals that can either:
- ⇰ Alter the structure of mycotoxins and produce less toxic compounds.
- ⇰ Destroy the mycotoxins.
During this degradation process, different microbes that produce various enzymes are utilized. Some of those enzymes are:
- ⇰ Aflatoxin oxidase produced by Armillariella tabescens (Cao et al., 2011).
- ⇰ Peroxidase produced by Pseudomonas species (Zaid, 2017).
- ⇰ Reductase produced by Mycobacterium smegmatis (Li et al., 2019).
Probiotics are also known to improve both zootechnical and biochemical indices of aquatic animals.
![]() A recent study by Sadeghi et al., (2020) tested the amelioration effect of probiotic bacteria L. rhamnosus against aflatoxicosis in rainbow trout for 4 weeks.
A recent study by Sadeghi et al., (2020) tested the amelioration effect of probiotic bacteria L. rhamnosus against aflatoxicosis in rainbow trout for 4 weeks.
⇰ At the end of the experiment, the groups fed diets containing aflatoxins showed a considerable increase in cortisol, alkaline phosphate enzyme, blood glucose, and mortality as well as significant reductions in chymotrypsin enzyme of the digestive system. However, upon inclusion of L. rhamnosus, amelioration effects in growth, survival, and enzyme balance were found.
![]() Similarly, a more recent study by Khalafalla et al. (2022) examined the effect of 106 CFU/mL per kg diet of probiotic bacteria Lactobacillus acidophilus against aflatoxicosis induced by 0.5-1 mg/kg aflatoxins on the growth, blood biochemistry and histopathology of grey mullet (Liza ramada) for 8 weeks.
Similarly, a more recent study by Khalafalla et al. (2022) examined the effect of 106 CFU/mL per kg diet of probiotic bacteria Lactobacillus acidophilus against aflatoxicosis induced by 0.5-1 mg/kg aflatoxins on the growth, blood biochemistry and histopathology of grey mullet (Liza ramada) for 8 weeks.
⇰ Significant reductions in growth rates, leukocytes, erythrocytes, haemoglobin, hematocrit, total protein and albumin in the blood, and cholesterol, as well as a considerable increase in liver enzymes, blood glucose, and cortisol, were reported from the groups fed only aflatoxin-containing feeds. Tissue histomorphology in aflatoxin groups also showed significant damage.
⇰ Groups supplemented with L. acidophilus and aflatoxins showed notable improvements in growth, blood biochemistry, antioxidant enzymes such as catalase and superoxide dismutase, and liver and intestinal histopathology.
![]() Another trial by Fang et al. (2020) also revealed that the probiotic bacteria Lactiplantibacillus pentosus improved aflatoxin-induced growth retardation, immunosuppression, and damage to the gut microbiota, and tissue histomorphology of pacific white shrimp.
Another trial by Fang et al. (2020) also revealed that the probiotic bacteria Lactiplantibacillus pentosus improved aflatoxin-induced growth retardation, immunosuppression, and damage to the gut microbiota, and tissue histomorphology of pacific white shrimp.
 To accelerate the detoxification process of mycotoxins in aquaculture a combination of microorganisms (probiotics preferably), degrading enzymes, and/or adsorbing agents can also be implemented (Guan et al., 2021). To accelerate the detoxification process of mycotoxins in aquaculture a combination of microorganisms (probiotics preferably), degrading enzymes, and/or adsorbing agents can also be implemented (Guan et al., 2021). |
![]() Huang et al. (2018b) found that applying a combination of Bacillus subtilis, Lactobacillus casei, and degrading enzyme produced by Aspergillus oryzae significantly accelerated the mycotoxin detoxification process.
Huang et al. (2018b) found that applying a combination of Bacillus subtilis, Lactobacillus casei, and degrading enzyme produced by Aspergillus oryzae significantly accelerated the mycotoxin detoxification process.
Plant-based feed additives against mycotoxicosis
Among the different strategies used to deal with the challenges of mycotoxins in aquaculture is the concept of plant-based feed additives.
Plants display a variety of essential oils, spices, herbs, and other extracted products that can play a noble role in mitigating the health effects caused by mycotoxins (Gowda et al., 2013).
They are considered eco-friendly, available, and affordable products to tackle not only mycotoxins but also many other problems in the feed and food industries (Iram et al., 2016).
![]() In recent years, the plant-derived compounds, coumarins and flavonoids have been found to inhibit the carcinogenic effect of aflatoxins (Lee et al., 2001).
In recent years, the plant-derived compounds, coumarins and flavonoids have been found to inhibit the carcinogenic effect of aflatoxins (Lee et al., 2001).
![]() Curcumin, the bioactive ingredient of turmeric, has also been reported to have a curative effect against aflatoxicosis (Ferreira et al., 2013).
Curcumin, the bioactive ingredient of turmeric, has also been reported to have a curative effect against aflatoxicosis (Ferreira et al., 2013).
![]() A study conducted by Vijayanandraj et al. (2014) examined the degradation capacity of various plant extracts and concluded that they also showed the highest aflatoxin degradation rate (about 98%).
A study conducted by Vijayanandraj et al. (2014) examined the degradation capacity of various plant extracts and concluded that they also showed the highest aflatoxin degradation rate (about 98%).
![]() It has also been reported that oil extracted from Chenopodium ambrosioides is an inhibitor of both aflatoxin and Fusarium mycotoxins (Sandosskumar et al., 2007).
It has also been reported that oil extracted from Chenopodium ambrosioides is an inhibitor of both aflatoxin and Fusarium mycotoxins (Sandosskumar et al., 2007).
![]() Abdelhiee et al. (2020) conducted a four-week study to examine how 0.5-1% of Moringa oleifera mitigates the adverse impacts induced by 1 mg/ kg of AFB1 on the growth, immune response, blood biochemical, and haematological indices of Nile tilapia.
Abdelhiee et al. (2020) conducted a four-week study to examine how 0.5-1% of Moringa oleifera mitigates the adverse impacts induced by 1 mg/ kg of AFB1 on the growth, immune response, blood biochemical, and haematological indices of Nile tilapia.
⇰ Necroscopic examinations of the groups fed with diets with aflatoxins but without moringa resulted in inferior growth performance (weight gain, specific growth rate, etc.), serum biochemistry (red and white blood cells, haemoglobin, hematocrit, and albumin), antioxidant enzymes (superoxide dismutase and catalase), as well as significant increase in liver enzymes.
⇰ In the histopathological examination of the groups fed with aflatoxin-containing feeds, vascular dilation, congestion, and necrotic liver, as well as lack of integrity in the intestines and lesions in the gills, were detected. In addition, when Moringa oleifera was supplemented with the diets of this experiment, significant improvements in growth, serum biochemical indices, tissue histomorphology, gene expression, and immune system were obtained.
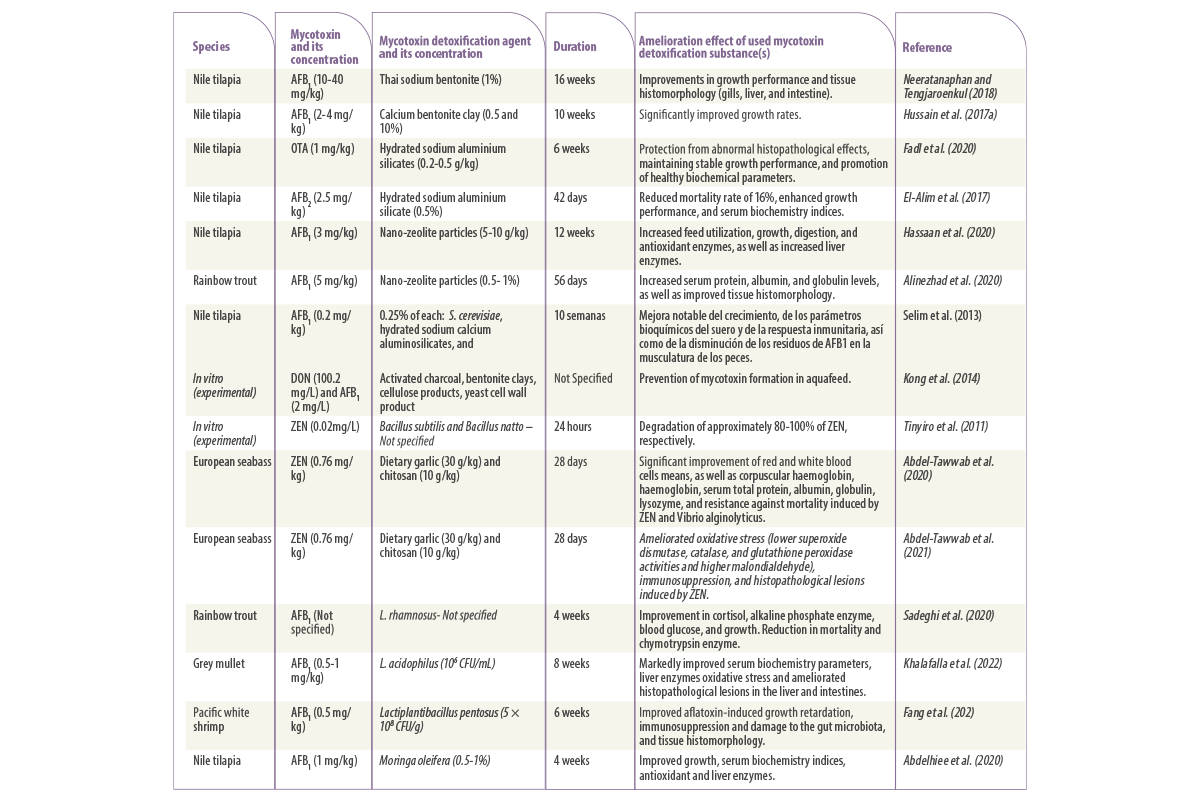
Table 1. Studies on the application of physical, biological, and microbial strategies to mitigate the adverse effects induced by mycotoxicosis in aquaculture.
Conclusions
Numerous mechanisms have been introduced to remove or reduce mycotoxins in aquaculture before or after the animals are exposed to them.
Chemical approaches entail the application of chemical compounds such as ozone, sodium hydroxide, ammonia, and different acids, which are mainly used to prevent mycotoxin formation in feed.
 Although chemical methods are effective in combating mycotoxins, they result in harmful effects since they leave residues and adversely affect the test and appearance of the meals. Although chemical methods are effective in combating mycotoxins, they result in harmful effects since they leave residues and adversely affect the test and appearance of the meals. |
Physical approaches involve mycotoxins with various methods like cleaning, UV lights, sorting, and sequestering or adsorbing agents. The latter, in particular, has become a magnet for many studies, and its effectiveness has been demonstrated.
⇰ Nevertheless, one major drawback of the physical approach is the poor adsorption capacity. Thus, combining different mineral adsorbing agents might be beneficial, but future research investigating this is necessary.
The microbiological approach is associated with implementing commensal bacteria, fungi, the enzymes they produce, and plant-based feed additives.
Plant-based feed additives are also highly recommended as they contain essential oils, herbs, spices, and other vital extracts that are effective to some extent in combating mycotoxins.
Microbes are inexpensive, eco-friendly, and most importantly, very efficient at degrading or adsorbing mycotoxins. However, compounds produced when mycotoxins are degraded may not be safe, and little is known about how such products may affect animals, a concern of the microbiological approach.
⇰ For this reason, studies aiming to discover the methods, dosage, time length of microbiological detoxification, and the safety of microbe-produced products are deemed necessary.
REFERENCES
Abdelhiee, E.Y., Elbialy, Z.I., Saad, A.H., Dawood, M.A., Aboubakr, M., El Nagar, S.H., El-Diasty, E.M., Salah, A.S., Saad, H.M. and Fadl, S.E., 2021. The impact of Moringa oleifera on the health status of Nile tilapia exposed to aflatoxicosis. Aquaculture, 533, 736110.
Abdel-Tawwab, M., Khalifa, E., Diab, A.M., Khallaf, M.A., Abdel-Razek, N. and Khalil, R.H., 2020. Dietary garlic and chitosan alleviated zearalenone toxic effects on performance, immunity, and challenge of European sea bass, Dicentrarchus labrax, to Vibrio alginolyticus infection. Aquaculture International, 28(2), 493-510.
Abdel-Tawwab, M., Khalil, R.H., Diab, A.M., Khallaf, M.A., Abdel-Razek, N., Abdel-Latif, H.M. and Khalifa, E., 2021. Dietary garlic and chitosan enhanced the antioxidant capacity, and immunity, and modulated the transcription of HSP70 and Cytokine genes in Zearalenone-intoxicated European seabass. Fish & Shellfish Immunology, 113, 35-41.
Alinezhad, S., Faridi, M., Falahatkar, B., Nabizadeh, R. and Davoodi, D., 2017. Effects of nanostructured zeolite and aflatoxin B1 in growth performance, immune parameters, and pathological conditions of rainbow trout Oncorhynchus mykiss. Fish & Shellfish Immunology, 70, 648-655.
Alinezhad, S., Tolouee, M., Kamalzadeh, A., Motalebi, A.A., Nazeri, M., Yasemi, M., Shams, G.M., Tolouei, R. and Razzaghi, A.M., 2011. Mycobiota and aflatoxin B1 contamination of rainbow trout (Oncorhynchus mykiss) feed with emphasis to Aspergillus section Flavi. Iranian Journal of Fisheries, 10(3), 363-374.
Arana, S., Dagli, M.L., Sabino, M., Tabata, Y.A., Rigolino, M.G. and Hernandez-Blazquez, F.J., 2011. Evaluation of the efficacy of hydrated sodium aluminosilicate in the prevention of aflatoxin-induced hepatic cancer in rainbow trout. Pesquisa veterinaria brasileira, 31, 751-755.
Avantaggiato, G., Havenaar, R. and Visconti, A., 2004. Evaluation of the intestinal absorption of deoxynivalenol and nivalenol by an in vitro gastrointestinal model, and the binding efficacy of activated carbon and other adsorbent materials. Food and Chemical Toxicology, 42(5), 817-824.
Battilani, C.P., 2008. Food mycology-a multifaceted approach to fungi and food. World Mycotoxin Journal, 1(2), 223-224.
Benvindo da Luz, A., & Lins, FAF., 2008. Rochas & minerais industriais: usos e especificações. ed. Rio de Janeiro, RJ: CETEM/MCT.
Brown, K.A., Mays, T., Romoser, A., Marroquin‐Cardona, A., Mitchell, N.J., Elmore, S.E. and Phillips, T.D., 2014. Modified hydra bioassay to evaluate the toxicity of multiple mycotoxins and predict the detoxification efficacy of a clay based sorbent. Journal of Applied Toxicology, 34(1), 40-48.
Cao, H., Liu, D., Mo, X., Xie, C. and Yao, D., 2011. A fungal enzyme with the ability of aflatoxin B1 conversion: Purification and ESI-MS/MS identification. Microbiological Research, 166(6), 475-483.
Čolović, R., Puvača, N., Cheli, F., Avantaggiato, G., Greco, D., Đuragić, O., Kos, J. and Pinotti, L., 2019. Decontamination of mycotoxin-contaminated feedstuffs and compound feed. Toxins, 11(11), p.617.
Conte, G., Fontanelli, M., Galli, F., Cotrozzi, L., Pagni, L. and Pellegrini, E., 2020. Mycotoxins in feed and food and the role of ozone in their detoxification and degradation: An update. Toxins, 12(8), p.486.
Daković, A., Kragović, M., Rottinghaus, G.E., Sekulić, Ž., Milićević, S., Milonjić, S.K. and Zarić, S., 2010. Influence of natural zeolitic tuff and organozeolites surface charge on sorption of ionizable fumonisin B1. Colloids and Surfaces B: Biointerfaces, 76(1), 272-278.
Di Gregorio, M.C., Neeff, D.V.D., Jager, A.V., Corassin, C.H., Carão, Á.C.D.P., Albuquerque, R.D., Azevedo, A.C.D. and Oliveira, C.A.F., 2014. Mineral adsorbents for prevention of mycotoxins in animal feeds. Toxin Reviews, 33(3), 125-135.
Diaz, D.E and Smith, T.K., 2005. Mycotoxin sequestering agents: practical tools for the neutralisation of mycotoxins. The Mycotoxin Blue Book: 323-339.
El-Alim, A., Galal, A.A., Mahmoud, S.H. and El-Sayed, W.A., 2017. Comparative ameliorative effect of hydrated sodium calcium aluminosilicate and Saccharomyces cerevisiae (Brewer’s yeast) against toxic impact of aflatoxin B1 in Oreochromis niloticus (Nile tilapia). Zagazig Veterinary Journal, 45(Supp. 1), 210-220.
Fadl, S.E., El-Shenawy, A.M., Gad, D.M., El Daysty, E.M., El-Sheshtawy, H.S. and Abdo, W.S., 2020. Trial for reduction of Ochratoxin A residues in fish feed by using nano particles of hydrated sodium aluminum silicates (NPsHSCAS) and copper oxide. Toxicon, 184, 1-9.
Fang, H., Wang, B., Jiang, K., Liu, M. and Wang, L., 2020. Effects of Lactobacillus pentosus HC-2 on the growth performance, intestinal morphology, immune-related genes and intestinal microbiota of Penaeus vannamei affected by aflatoxin B1. Aquaculture, 525, 735289.
Ferreira, F.D., Kemmelmeier, C., Arrotéia, C.C., da Costa, C.L., Mallmann, C.A., Janeiro, V., Ferreira, F.M.D., Mossini, S.A.G., Silva, E.L. and Machinski Jr, M., 2013. Inhibitory effect of the essential oil of Curcuma longa L. and curcumin on aflatoxin production by Aspergillus flavus Link. Food Chemistry, 136(2), 789-793.
Fruhauf, S., Schwartz, H., Ottner, F., Krska, R. and Vekiru, E., 2012. Yeast cell based feed additives: studies on aflatoxin B1 and zearalenone. Food Additives & Contaminants: Part A, 29(2), 217-231.
Ghasemi, Z., Sourinejad, I., Kazemian, H. and Rohani, S., 2018. Application of zeolites in aquaculture industry: a review. Reviews in Aquaculture, 10(1), 75-95.
Gowda, N.K.S., Swamy, H.V.L.N. and Mahajan, P., 2013. Recent advances for control, counteraction and amelioration of potential aflatoxins in animal feeds. In: Aflatoxins-Recent Advances and Future Prospects, Ed: Mehdi Razzaghi Abyaneh, Chapter 6., pp.129-140. Published by InTech, Rijeka, Croatia.
Guan, Y., Chen, J., Nepovimova, E., Long, M., Wu, W. and Kuca, K., 2021. Aflatoxin detoxification using microorganisms and enzymes. Toxins, 13(1), p.46.
Hassaan, M.S., Nssar, K.M., Mohammady, E.Y., Amin, A., Tayel, S.I. and El Haroun, E.R., 2020. Nano-zeolite efficiency to mitigate the aflatoxin B1 (AFB1) toxicity: Effects on growth, digestive enzymes, antioxidant, DNA damage and bioaccumulation of AFB1 residues in Nile tilapia (Oreochromis niloticus). Aquaculture, 523, 735123.
Haskard, C.A., El-Nezami, H.S., Kankaanpää, P.E., Salminen, S. and Ahokas, J.T., 2001. Surface binding of aflatoxin B1 by lactic acid bacteria. Applied and environmental microbiology, 67(7), 3086-3091.
Huang, W.; Chang, J.; Wang, P. 2018b. Effect of the combined compound probiotics with mycotoxin-degradation enzyme on detoxifying aflatoxin B1 and zearalenone. J. Toxicol. Sci. 43, 377–385.
Hussain, D. and Mateen, A., 2017b. Alleviation of aflatoxin-B1 toxicity by using clay adsorbent in Nile tilapia (Oreochromis niloticus) diets. Pakistan Journal of Zoology, 49(2), 425-431.
Hussain, D., Mateen, A. and Gatlin III, D.M., 2017a. Alleviation of aflatoxin B1 (AFB1) toxicity by calcium bentonite clay: Effects on growth performance, condition indices and bioaccumulation of AFB1 residues in Nile tilapia (Oreochromis niloticus). Aquaculture, 475, 8-15.
Hussain, D., 2018. Effect of aflatoxins in aquaculture: use of bentonite clays as promising remedy. Turkish Journal of Fisheries and Aquatic Sciences, 18(8), 1009-1016.
Huwig, A., Freimund, S., Käppeli, O. and Dutler, H., 2001. Mycotoxin detoxication of animal feed by different adsorbents. Toxicology letters, 122(2), 179-188.
Ji, J. and Xie, W., 2021. Removal of aflatoxin B1 from contaminated peanut oils using magnetic attapulgite. Food Chemistry, 339, 128072.
Iram, W., Anjum, T., Iqbal, M., Ghaffar, A. and Abbas, M., 2016. Structural elucidation and toxicity assessment of degraded products of aflatoxin B1 and B2 by aqueous extracts of Trachyspermum ammi. Frontiers in Microbiology, 7, 346.
Kabak, B., Dobson, A.D. and Var, I.I.L., 2006. Strategies to prevent mycotoxin contamination of food and animal feed: a review. Critical reviews in food science and nutrition, 46(8), 593-619.
Kannewischer, I., Arvide, M.G.T., White, G.N. And Dixon, J.B., 2006. Smectite clays as adsorbents of aflatoxin B1: Initial steps. Clay Science, 12 (Supp 2), 199-204
Karlovsky, P., Suman, M., Berthiller, F., De Meester, J., Eisenbrand, G., Perrin, I., Oswald, I.P., Speijers, G., Chiodini, A., Recker, T. and Dussort, P., 2016. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Research, 32(4), 179-205.
Khalafalla, M.M., Zayed, N.F., Amer, A.A., Soliman, A.A., Zaineldin, A.I., Gewaily, M.S., Hassan, A.M., Van Doan, H., Tapingkae, W. and Dawood, M.A., 2022. Dietary Lactobacillus acidophilus ATCC 4356 Relieves the Impacts of Aflatoxin B1 Toxicity on the Growth Performance, Hepatorenal Functions, and Antioxidative Capacity of Thinlip Grey Mullet (Liza ramada)(Risso 1826). Probiotics and Antimicrobial Proteins, 14(1), 189-203.
Kolosova, A. and Stroka, J., 2011. Substances for reduction of the contamination of feed by mycotoxins: A review. World Mycotoxin Journal, 4(3), 225-256.
Kong, C., Shin, S.Y. and Kim, B.G., 2014. Evaluation of mycotoxin sequestering agents for aflatoxin and deoxynivalenol: an in vitro approach. SpringerPlus, 3(1), 346.
Lee, S.E., Campbell, B.C., Molyneux, R.J., Hasegawa, S. and Lee, H.S., 2001. Inhibitory effects of naturally occurring compounds on aflatoxin B1 biotransformation. Journal of Agricultural and Food Chemistry, 49(11), 5171 5177.
Li, C.H., Li, W.Y., Hsu, I.N., Liao, Y.Y., Yang, C.Y., Taylor, M.C., Liu, Y.F., Huang, W.H., Chang, H.H., Huang, H.L. and Lo, S.C., 2019. Recombinant aflatoxin-degrading F420H2-dependent reductase from mycobacterium smegmatis protects mammalian cells from aflatoxin toxicity. Toxins, 11(5), 259.
Nathanail, A.V., Gibson, B., Han, L., Peltonen, K., Ollilainen, V., Jestoi, M. and Laitila, A., 2016. The lager yeast Saccharomyces pastorianus removes and transforms Fusarium trichothecene mycotoxins during fermentation of brewer’s wort. Food Chemistry, 203, 448-455.
Neeratanaphan, L. and Tengjaroenkul, B., 2018. Protective effects of Thai bentonite on aflatoxin B1 contaminated in diet of tilapia fish. Livestock Research for Rural Development, 30(8), 152.
Rahaie, S., Emam‐Djomeh, Z., Razavi, S.H. and Mazaheri, M., 2012. Evaluation of aflatoxin decontaminating by two strains of Saccharomyces cerevisiae and Lactobacillus rhamnosus strain GG in pistachio nuts. International Journal Of Food Science & Technology, 47(8), 1647-1653.
Sadeghi, N., Bahadori, R., Ojagh, S.M. and Salamroodi, E., 2020. Effect of dietary Lactobacillus rhamnosus on blood biochemical indices and some digestive enzymes activity in rainbow trout (Oncorhynchus mykiss) fed with aflatoxin B1 infected diet. Journal of Animal Environment, 12(2), 151-160.
Sandosskumar, R., Karthikeyan, M., Mathiyazhagan, S., Mohankumar, M., Chandrasekar, G. and Velazhahan, R., 2007. Inhibition of Aspergillus flavus growth and detoxification of aflatoxin B1 by the medicinal plant zimmu (Allium sativum L.× Allium cepa L.). World Journal of Microbiology and Biotechnology, 23(7), 1007-1014.
Sava, S.C., Pogurschi, E., Bahaciu, G.V., Dragomir, N. and Nicolae, C.G., 2019. The effects of clinoptilolite from feed upon fish rearing and water quality. Current Trends in Natural Sciences, 8(16), 212-219.
Selim, K.M., El-hofy, H. and Khalil, R.H., 2014. The efficacy of three mycotoxin adsorbents to alleviate aflatoxin B1-induced toxicity in Oreochromis niloticus. Aquaculture International, 22(2), 523-540.
Shaban, M., AbuKhadra, M.R., Nasief, F.M. and El-Salam, A., 2017. Removal of ammonia from aqueous solutions, ground water, and wastewater using mechanically activated clinoptilolite and synthetic zeolite-a: kinetic and equilibrium studies. Water, Air, & Soil Pollution, 228(11), 1-16.
Taheur, F.B., Fedhila, K., Chaieb, K., Kouidhi, B., Bakhrouf, A. and Abrunhosa, L., 2017. Adsorption of aflatoxin B1, zearalenone and ochratoxin A by microorganisms isolated from Kefir grains. International Journal of Food Microbiology, 251, 1-7.
Tapia Salazar, M., García-Pérez, O.D., Nieto López, M., Ricque Marie, D., Villarreal Cavazos, D. and Cruz Suárez, L.E., 2010. Mycotoxins in aquaculture: Occurrence in feeds components and impact on animal performance. In: Avances en Nutrición Acuícola X-Memorias del Décimo Simposio Internacional de Nutrición Acuícola (pp.8-10).
Tinyiro, S.E., Wokadala, C., Xu, D. and Yao, W., 2011. Adsorption and degradation of zearalenone by bacillus strains. Folia Microbiologica, 56(4), 321-327.
Vekiru, E., Fruhauf, S., Sahin, M., Ottner, F., Schatzmayr, G. and Krska, R., 2007. Investigation of various adsorbents for their ability to bind aflatoxin B1. Mycotoxin Research, 23(1), 27-33.
Vijayanandraj, S., Brinda, R., Kannan, K., Adhithya, R., Vinothini, S., Senthil, K., Chinta, R.R., Paranidharan, V. and Velazhahan, R., 2014. Detoxification of aflatoxin B1 by an aqueous extract from leaves of Adhatoda vasica Nees. Microbiological Research, 169(4), 294-300.
Vila-Donat, P., Marín, S., Sanchis, V. and Ramos, A.J., 2018. A review of the mycotoxin adsorbing agents, with an emphasis on their multi-binding capacity, for animal feed decontamination. Food and Chemical Toxicology, 114, 246-259.
Wagner, S., Gondikas, A., Neubauer, E., Hofmann, T. and von der Kammer, F., 2014. Spot the difference: engineered and natural nanoparticles in the environment—release, behavior, and fate. Angewandte Chemie International Edition, 53(46), 12398-12419.
Wielogórska, E., MacDonald, S. and Elliott, C.T., 2016. A review of the efficacy of mycotoxin detoxifying agents used in feed in light of changing global environment and legislation. World Mycotoxin Journal, 9(3), 419-433.
Yu, Y., Shi, J., Xie, B., He, Y., Qin, Y., Wang, D., Shi, H., Ke, Y. and Sun, Q., 2020. Detoxification of aflatoxin B1 in corn by chlorine dioxide gas. Food Chemistry, 328, 127121.
Zahran, E., Risha, E., Hamed, M., Ibrahim, T. and Palić, D., 2020. Dietary mycotoxicosis prevention with modified zeolite (Clinoptilolite) feed additive in Nile tilapia (Oreochromis niloticus). Aquaculture, 515, 734562.
Zaid, A.M.A., 2017. Biodegradation of aflatoxin by peroxidase enzyme produced by local isolate of Pseudomonas sp. Int J Sci Res Manag, 5, 7456-7467.
Zhou, J., Tang, L., Wang, J. and Wang, J.S., 2018. Aflatoxin B1 disrupts gut microbial metabolisms of short-chain fatty acids, long-chain fatty acids, and bile acids in male F344 rats. Toxicological sciences, 164(2), 453-464.
Zychowski, K.E., Hoffmann, A.R., Ly, H.J., Pohlenz, C., Buentello, A., Romoser, A., Gatlin, D.M. and Phillips, T.D., 2013. The effect of aflatoxin-B1 on red drum (Sciaenops ocellatus) and assessment of dietary supplementation of NovaSil for the prevention of aflatoxicosis. Toxins, 5(9), 1555-1573.




 Micotoxicosis prevention
Micotoxicosis prevention